Antibacterial Activity of the Mucus Extract from the Giant African Snail (Lissachatina fulica) and Golden Apple Snail (Pomacea canaliculata) Against Pathogenic Bacteria Causing Skin Diseases
DOI:
https://doi.org/10.58837/tnh.19.2.150872Keywords:
Lissachatina, Pomacea, antimicrobial, snail slime, pathogensAbstract
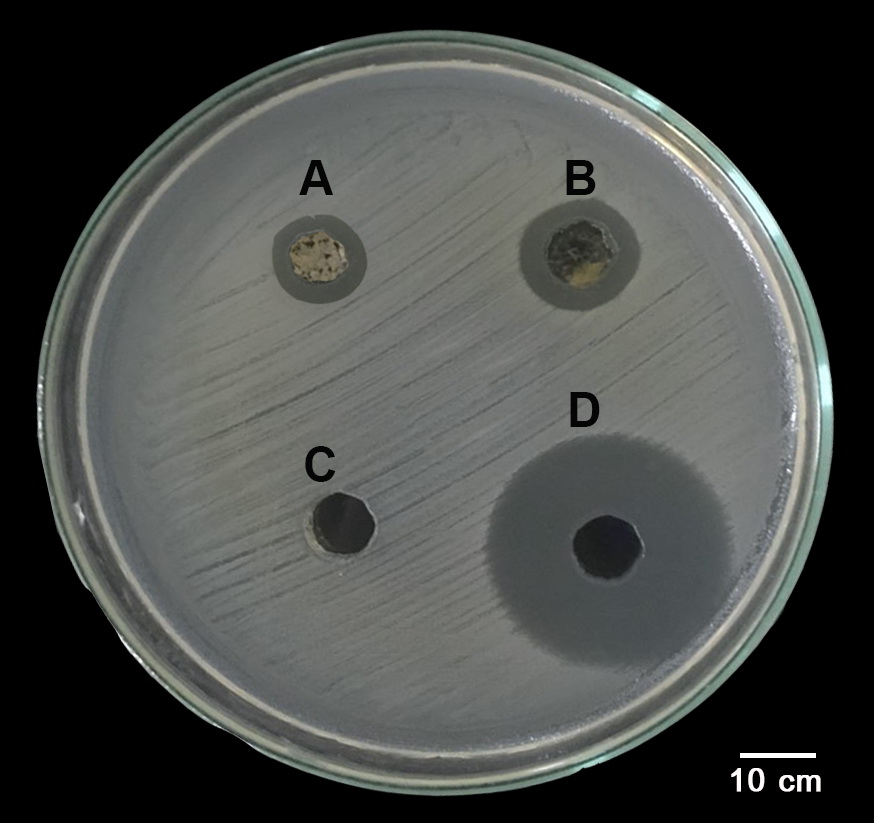
There have been many cases of snails reported to be agricultural pests in Thailand, including the important invasive pests, giant African snail, Lissachatina fulica, and the golden apple snail, Pomacea canaliculata. These snails have rich mucus that covers their surface, which may serve in preventing moisture evaporation, reducing friction and providing resistant to infection by microorganisms. In this study, the antibacterial activity of aqueous extracts of L. fulica and P. canaliculata mucus were tested against four strains of Gram-positive bacteria, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Staphylococcus epidermidis and Corynebacterium sp. Thirty adult snail samples of both L. fulica and P. canaliculata were collected, snail mucus was harvested, and a crude aqueous extract of the mucus (CME) prepared. The in vitro antibacterial activity of each CME was evaluated by the agar well diffusion method, while the broth dilution method was used to determine its minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). CME from both L. fulica and P. canaliculata displayed antibacterial activity against all four strains of Gram-positive bacteria in the agar well diffusion assay. In the broth dilution assay, CME from L. fulica showed weak activity against all four bacterial strains, being highest against S. aureus and MRSA (MIC 12.5 µg/ml; MBC >50 µg/ml), followed by S. epidermidis and Corynebacterium sp. (MIC 25 µg/ml; MBC >50 µg/ml); however, that from P. canaliculata showed no antibacterial activity against these bacteria. Therefore, CMEs from these two snail species were somewhat effective against these pathogens, and might be useful for human health-related applications in the future, following further fractionation to isolate the active components and determination of their optimal concentrations, and whether or not they act synergistically.
References
Adikwu, M. and U. Alozie, B., 2007. Application of snail mucin dispersed in detarium gum gel in wound healing. Scientific Research and Essay, 2: 195–198.
Berniyanti, T., Waskito, E.B. and Suwarno, S., 2015. Biochemival Characterization of an Antibactrial Glycoprotein from Achatina fulica ferussac Snail Mucus Local Isolate and Their Implication on Bacterial Dental Infection, Indonesian Journal of Biotechnology, 12(1): 943–951 pp. https://doi.org/ 10.22146/ijbiotech.7765.
Bhattacharyya, B., Das, M., Mishra, H. and Nath, D.J., 2014. Bioecology and management of giant African snail , Achatina fulica ( Bowdich ), International Journal of Plant Protection, 7(2): 476–481.
Chandaragi, M., 2014. Integrated management of giant african snail, Achatina fulica (Ferussac) (Stylommataphora:Achatinidae) in agriculture and horticulture ecosystems 202 pp.
Cilia, G. and Fratini, F., 2018. Antimicrobial properties of terrestrial snail and slug mucus., Journal of complementary & integrative medicine, 15(3): 1–10. https://doi.org/10.1515/ jcim-2017-0168.
Dwi-Nugrahananto, H., Kriswandini, I. and R, E., 2014. Antimicrobial proteins of Snail mucus (Achatina fulica) against Streptococcus mutans and Aggregatibacter actinomycetemcomitans, Dental Journal, 47: 31–36. https://doi.org/10.204 73/ j.djmkg.v47.i1.
Ehara, T., Kitajima, S., Kanzawa, N., Tamiya, T. and Tsuchiya, T., 2002. Antimicrobial action of achacin is mediated by L-amino acid oxidase activity., FEBS letters, 531(3): 509–512.
Etim, L., Aleruchi, C. and Obande, G., 2016. Antibacterial Properties of Snail Mucus on Bacteria Isolated from Patients with Wound Infection, British Microbiology Research Journal, 11(2): 1–9. https://doi.org/10.9734/BMRJ/2016/ 21731.
Fagbuaro, O., Oso, J.A., Edward, J.B. and Ogunleye, R.F., 2006. Nutritional status of four species of giant land snails in Nigeria, Journal of Zhejiang University. Science. B, 7(9): 686–689. https://doi. org/10.1631/jzus.2006.B0686.
Halcon, L. and Milkus, K., 2004. Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial., American journal of infection control, 32(7): 402–408. https://doi.org /10.1016/S0196655304003657.
Hayes, K.A., Joshi, R.C., Thiengo, S.C. and Cowie, R.H., 2008. Out of South America: multiple origins of non-native apple snails in Asia, Diversity and Distributions, 14(4): 701–712. DOI: https://doi.org/10.1111/j.1472-4642.2008.00483.x.
IBM Corp, 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk (ed.). New York: IBM Corp.
Iguchi, S.M., Aikawa, T. and Matsumoto, J.J., 1982. Antibacterial activity of snail mucus mucin., Comparative biochemistry and physiology, 72(3): 571–574.
Ito, S., Shimizu, M., Nagatsuka, M., Kitajima, S., Honda, M., Tsuchiya, T. and Kanzawa, N., 2011. High molecular weight lectin isolated from the mucus of the giant African snail Achatina fulica., Bioscience, biotechnology, and biochemistry, 75(1): 20–25. https://doi.org/10.1271/bbb.100389.
Jantakee, K. and Tragoolpua, Y., 2015. Activities of different types of thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals, Biological Research, 48: 1–11. https://doi.org/10.1186/0717-6287-48-4.
Otsuka-Fuchino, H., Watanabe, Y., Hirakawa, C., Tamiya, T., Matsumoto, J.J. and Tsuchiya, T., 1992. Bactericidal action of a glycoprotein from the body surface mucus of giant African snail., Comparative biochemistry and physiology, 101(3): 607–613 pp.
Pitt, S.J., Graham, M.A., Dedi, C.G., Taylor-Harris, P.M. and Gunn, A., 2015. Antimicrobial properties of mucus from the brown garden snail Helix aspersa., British journal of biomedical science, 72(4): 174–81.
Roughton, B.C., Iyer, L.K., Bertelsen, E., Topp, E.M. and Camarda, K. V, 2013. Protein aggregation and lyophilization: Protein structural descriptors as predictors of aggregation propensity, Computers & chemical engineering, 58: 369–377.
Salleh, N.H.M., Arbain, D., Daud, M.Z.M., Pilus, N. and Nawi, R., 2012. Distribution and Management of Pomacea Canaliculata in the Northern Region of Malaysia: Mini Review, APCBEE Procedia, 2: 129–134. https://doi.org/10.1016/j.apcbee. 2012. 06.024.
Santana, W.A., Melo, C.M. de, Cardoso, J.C., Pereira-Filho, R.N., Rabelo, A.S., Reis, F.P. and Albuquerque-Júnior, R.L.C. de, 2012. Assessment of Antimicrobial Activity and Healing Potential of Mucous Secretion of Achatina fulica, International Journal of Morphology, 30(2): 365–373. https://doi.org/10.4067/S0717-95022012000 200001.
Takeichi, M., Hirai, Y. and Yusa, Y., 2007. A water-borne sex pheromone and trail following in the apple snail, Pomacea canaliculata, Journal of Molluscan Studies, 73(3): 275–278. https://doi. org/ 10.1093/mollus/eym027.
Ulagesan, S. and Kim, H., 2018. Antibacterial and Antifungal Activities of Proteins Extracted from Seven Different Snails, Applied Sciences, 8(8): 1362. https://doi.org/10.3390/app8081362.
Wasiq Hidayat, J. and Parman, S., 2015. Utilization digestive tract of golden snail (Pomacea canaliculata) as lytic enzyme for protoplast isolation Pichia manshurica DUCC-Y15, European Journal of Experimental Biology, 5(10): 49–53.
Yang, T.B., Wu, Z.D. and Lun, Z.R., 2013. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonensis: its introduction, spread, and control in China, Hawaii Journal of Medicine and Public Health, 72: 23–25.
Downloads
Published
How to Cite
Issue
Section
License
Chulalongkorn University. All rights reserved. No part of this publication may be reproduced, translated, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior written permission of the publisher











