Multiple Tail-like Structure Induced by Nitrogen Fertilisers in Hoplobatrachus rugulosus Embryos
DOI:
https://doi.org/10.58837/tnh.20.1.193568Keywords:
ammonium sulfate, urea, Hoplobatrachus rugulosus, teratogenesis, multiple tail-like structureAbstract
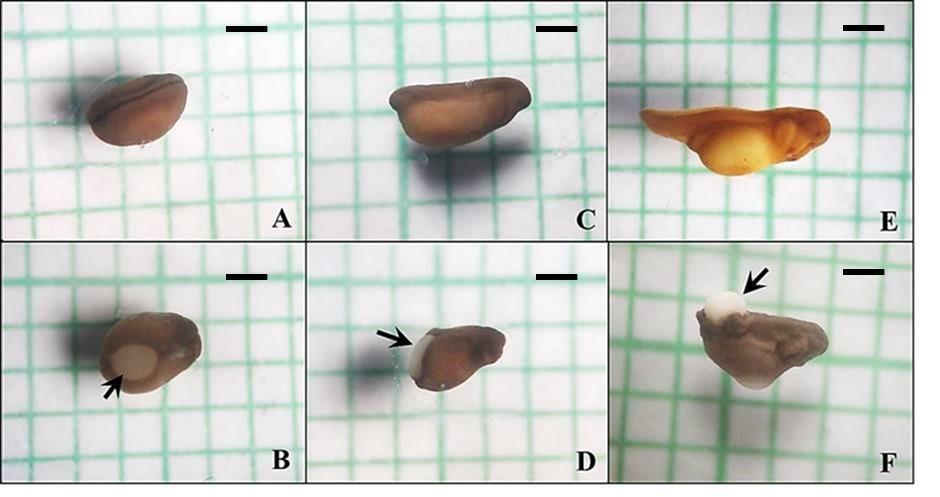
Agrochemical contamination is claimed as one of the most important factors in amphibian decline. Although many researchers previously focused on pesticide toxicity, fertiliser toxicity is also a prominent issue due to the massive amounts applied to fields by farmers each year. Therefore, the present study aimed to investigate acute toxicity of nitrogen fertilisers (ammonium sulphate and urea) on mortality and development of gastrula and neurula in the East Asian bull frog (Hoplobatrachus rugulosus) using the frog embryo teratogenesis assay. The results revealed lethality, malformation, and negative developmental effects induced by ammonium sulphate and urea fertilisers in H. rugulosus gastrulae and neurulae. Ammonium sulphate produced more severe effects on H. rugulosus embryos compared to urea for all measures in the same stage of embryos. Gastrulae were more sensitive to the exposure of the two nitrogen fertilisers. Moreover, the present study is the first report of a multiple tail-like structure caused by fertilisers in frog embryos. The two fertilisers also produced oedema and kinking of tail and body in both stages. This study suggests that the abnormality occurred due to interference with cell movements during gastrulation.
References
Banerjee, T.K. and Paul, V.I. 1993. Estimation of acute toxicity of ammonium sulfate to the freshwater catfish, Heteropneustes fossilis. II. A histopathological analysis of the epidermis. Biomedical and Environmental Sciences, 6: 45-58.
Beck, C.W. and Slack, J.M.W. 1998. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mechanisms of Development, 72(1): 41-52.
Boğa, A., Bİnokay, S. and Sertdemİr, Y. 2008. The toxicity and teratogenicity of gibberellic acid (GA 3) based on the frog embryo teratogenesis assay-Xenopus (FETAX). In Vitro Cellular and Developmental Biology, 33: 181-188.
Capkin, E., Kayis, S., Boran, H. and Altinok, I. 2010. Acute toxicity of some agriculture fertilizers to rainbow trout. Turkish Journal of Fisheries and Aquatic Sciences, 25: 19-25.
Devaraj, S., Arulprakasam, C., Kandhan, A.P., Neelamegam, K. and Kalaiselvan, R. 2014. Toxicological effects of ammonia on gills of Cyprinus carpio var. communis (Linn.). Journal of Coastal Life Medicine, 2(2): 94-98.
Edelman, G.M. 1984. Cell adhesion and morphogenesis: the regulator hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 81: 1460-1464.
Essien-ibok, M.A., Asuquo, I.E. and Ekpo, I.E. 2015. The assessment of acute toxicity of urea fertilizer against Heterobranchus bidorsalis fingerlings. Global Journal of Fisheries and Aquaculture, 2(5): 169-176.
Fabro, S., Shull, G. and Brown, N.A. 1982. The relative teratogenic index and teratogenic potency: proposed components of developmental toxicity risk assessment. Teratogenesis, Carcinogenesis, and Mutagenesis, 2(1): 61-76.
Food and Agriculture Organization of the United Nations (FAO). 2016. FAOSTAT Database. Retrieved Mar 16, 2016, from https://faostat.fao.org/site/424/DesktopDefault.aspx?PageID=424.
Fort, D.J., Propst, T.L., Stover, E.L., Helgen, J.C., Levey, R.B., Gallagher, K. and Burkhart, J.G. 1999. Effects of pond water, sediment, and sediment extracts from Minnesota and Vermont, USA, on early development and metamorphosis of Xenopus. Environmental Toxicology and Chemistry, 18(10): 2305-2315.
Fryday, S. and Thompson, H. 2012. Toxicity of Pesticides to Aquatic and Terrestrial Life Stages of Amphibians and Occurrence, Habitat Use and Exposure of Amphibian Species in Agricultural Environments. Supporting Publications, Food and Environment research Agency, York, 348 pp.
Gardner, D.K. and Lane, M. 1993. Amino acids and ammonium regulate mouse embryo development in culture. Biology of Reproduction, 48(2): 377-385.
Gilbert, S.F. 2006. Developmental Biology, 6th Ed. Sinauer Associates, Sunderland, 709 pp.
Glacken, M.W., Adema, E. and Sinskey, A.J. 1988. Mathematical description of hybridoma culture kinetics: I. Initial metabolic rates. Biotechnology and Bioengineering, 32: 491-506.
Glacken, M.W., Fleischaker, R.J. and Sinskey, A.J. 1986. Reduction of waste product excretion via nutrient control: possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells. Biotechnology and Bioengineering, 28: 1376-1389.
Golchin, A., Asadpour, R., Roshangar, L. and Jafari-jozani, R. 2015. The effect of ammonium chloride concentration in in vitro maturation culture on ovine embryo development. Journal of Reproduction and Infertility, 17(3): 144-150.
Gosner, K.L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3): 183-190.
Greulich, K. and Pflugmacher, S. 2003. Differences in susceptibility of various life stages of amphibians to pesticide exposure. Aquatic Toxicology, 65(3): 329-336.
Gupta, T. 2016. Effect of short term exposure to urea on catfish (Heteropneustes fossilis) a light microscopic study of the gills. European Journal of Pharmaceutical and Medical Research, 3(1): 161–163.
Hill, A.J., Bello, S.M., Prasch, A.L., Peterson, R.E. and Heideman, W. 2004. Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicological Sciences, 78(1): 78-87.
Holtfreter, J. 1931. Uber die aufzucht isolierter teile des amphibian keimes II. Archiv für Entwick-lungsmechanik der Organismen, 124: 404–465.
Houlahan, J.E., Findlay, C.S., Schmidt, B.R., Meyer, A.H. and Kuzmin, S.L. 2000. Quantitative evidence for global amphibian population declines. Nature, 404: 752-755.
Husk, B.R., Anderson, B.C., Whalen, J.K. and Sanchez, J.S. 2017. Reducing nitrogen contamination from agricultural subsurface drainage with denitrification bioreactors and controlled drainage. Biosystems Engineering, 153: 52-62.
Johnson, P.T.J., Lunde, K.B., Ritchie, E.G. and Launer, A.E. 1999. The effect of trematode infection on amphibian limb development and survivorship. Science, 284: 802–804.
Lane, M. and Gardner, D.K. 2003. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biology of Reproduction, 69(4): 1109-1117.
Lien, N.T., Adriaens, D. and Janssen, C.R. 1997. Morphological abnormalities in African catfish (Clarias gariepinus) larvae exposed to malathion. Chemosphere, 35: 1475–1486.
Lin, C.C., Hui, M.N.Y. and Cheng, S.H. 2007. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicology and Applied Pharmacology, 222(2): 159–168.
Marco, A., Cash, D., Belden, K. and Blaustein, A.R. 2001. Sensitivity to urea fertilization in three amphibian species. Archives of Environmental Contamination and Toxicology, 40: 406-409.
Maden, M. and Hind, M. 2003. Retinoic acid, a regeneration-inducing molecule. Developmental Dynamics, 226(2): 237-244.
Mann, R.M., Hyne, R.V., Choung, C.B. and Wilson, S.P. 2009. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environmental Pollution, 157(11): 2903-2927.
Miller, W.M., Wilke, C.R. and Blanch, H.W. 1988. Transient responses of hybridoma cells to lactate and ammonia pulse and step changes in continuous culture. Bioprocess Engineering, 3: 113-122.
Monroy, A. and Moscona, A.A. 1979. Introductory Concepts in Developmental Biology. University of Chicago Press, Chicago, 252 pp.
Norenberg, M.D., Rama-Rao, K.V. and Jayakumar, A.R. 2009. Signaling factors in the mechanism of ammonia neurotoxicity. Metabolic Brain Disease, 24: 103-117.
Ofojekwu, P.C., Nwani, C.D. and Ihere, R.E. 2008. Acute toxicity of urea fertilizer to Tilapia zilli fingerlings. Bio-Research, 6(1): 298-300.
Ozturk, S.S., Riley, M.R. and Palsson, B.O. 1992. Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production. Biotechnology and Bioengineering, 39: 418-431.
Parsons, M.J., Pollard, S.M., Feldman, B., Coutinho, P., Hirst, E.M. and Stemple, D.L. 2002. Zebrafish mutants identify an essential role for laminins in notochord formation. Development, 129(13): 3137-3146.
Raidal, S.R. and Jaensch, S.M. 2006. Acute poisoning of silver gulls (Larus novaehollandiae) following urea fertilizer spillage. Avian Pathology, 35(1): 38-41.
Ram, R.N. and Sathyanesan, A.G. 1987. Histopathological changes in liver and thyroid of the teleost fish, Channa punctatus (Bloch), in response to ammonium sulfate fertilizer treatment. Ecotoxicology and Environmental Safety, 13(2): 185-190.
Reuveny, S., Velez, D., Macmillan, J.D. and Miller, L. 1986. Factors affecting cell growth and monoclonal antibody production in stirred reactors. Journal of Immunological Methods, 86: 53-59.
Saxena, P., Kumar, P., Nagpure, N.S., Kumar, D. and Mathur, P.K. 2016. Evaluation of lethal concentration of ammonium sulphate to freshwater fish Mystus vitatus (BLOCH). Journal of Biological Sciences, 2(3): 1-5.
Schuytema, G.S. and Nebeker, A.V. 1999. Comparative toxicity of ammonium and nitrate compounds to Pacific treefrog and African clawed frog tadpoles. Environmental Toxicology and Chemistry, 18(10): 2251-2257.
Sessions, S.K., Franssen, R.A. and Horner, V.L. 1999. Morphological clues from multilegged frogs: are retinoids to blame?. Science, 284: 800-802.
Sessions, S.K. and Ruth, S.B. 1990. Explanation of naturally occurring supernumerary limbs in amphibians. Journal of Experimental Zoology, 254: 38-47.
Stratford, T., Horton, C. and Maden, M. 1996. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Current Biology, 6: 1124-1133.
Stratford, T., Logan, C., Zile, M. and Maden, M. 1999. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mechanisms of Development, 81: 115-125.
Tabin, C.J. 1991. Retinoids, homeoboxes, and growth factors: Toward molecular models for limb development. Cell, 66: 199-217.
Tchounwou, P.B., Englande, A.J. Jr. and Malek, E.A. 1991. Toxicity evaluation of ammonium sulphate and urea to three developmental stages of freshwater snails. Archives of Environmental Contamination and Toxicology, 21: 359-364.
Wijer, P., Watt, P.J. and Oldham, R.S. 2003. Amphibian decline and aquatic pollution: effects of nitrogenous fertiliser on survival and development of larvae of the frog Rana temporaria. Applied Herpetology, 1: 3-12.
Wright, P.A. 1995. Review nitrogen excretion: three end products, many physiological roles. The Journal of Experimental Biology, 198: 273-281.
Zhang, Y., Shao, M., Wang, L., Liu, Z., Gao, M., Liu, C. and Zhang, H. 2010. Ethanol exposure affects cell movement during gastrulation and induces split axes in zebrafish embryos. International Journal of Developmental Neuroscience, 28(4): 283-288.
Downloads
Published
How to Cite
Issue
Section
License
Chulalongkorn University. All rights reserved. No part of this publication may be reproduced, translated, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior written permission of the publisher











