Neuroprotective effect of Alpinia galanga against neurodegeneration in the rat hippocampus induced by kainic acid
Keywords:
Alpinia galanga, kainic acid, neurodegeneration, glial fibrillary acidic proteinAbstract
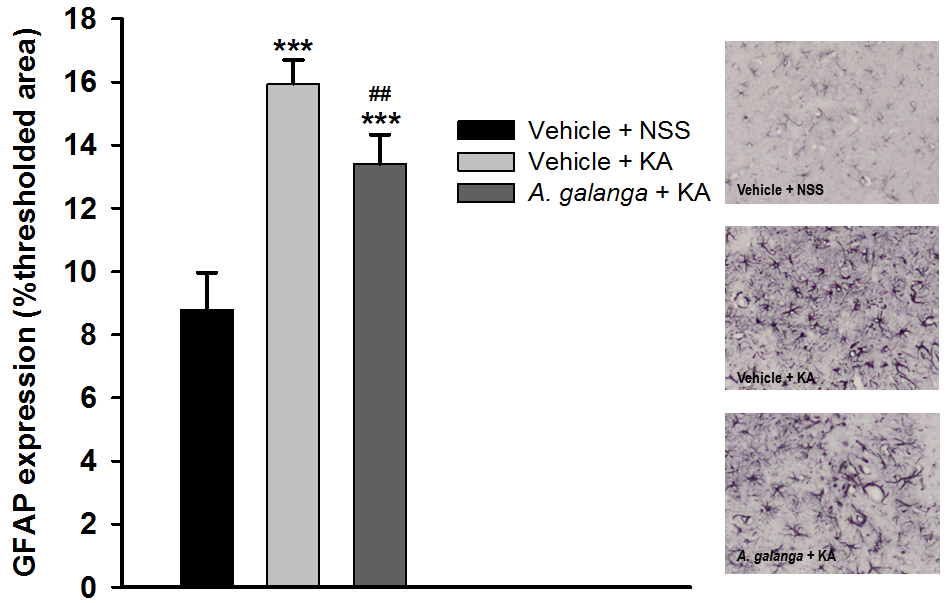
Injection of kainic acid (KA) can induce neurodegeneration and epilepsy in the experimental study. Alpinia galanga (A. galanga) exerted various biological activities including potent antioxidant and anti-inflammatory activities. However, the neuroprotective effect in KA model has not been elucidated. Thus, the authors examined the neuroprotective effect of A. galanga extract in the hippocampal rat’s brain. Young adult male Wistar rats were randomly assigned into three groups including vehicle (sodium carboxymethyl cellulose, NaCMC) plus normal saline injection, vehicle plus KA injection and the A. galanga extract (200 mg/kg BW) plus KA injection. The rats were treated with either A. galanga extract or vehicle for two weeks before and two weeks after the injection of KA (0.8 µg) into the right hippocampus. At the end of the experiment, the rats were sacrificed and their brains were collected to determine the expression of glial fibrillary acidic protein (GFAP) and neuron density in the hippocampal CA3 subregion. KA injection into the hippocampus significantly induced neuronal loss and increased the expression of GFAP. The A. galanga extract treatment significantly attenuated the neurodegeneration induced by KA, as evident by the significant increase of neuron density but decrease the percent of GFAP immunoreactive expression compared with the vehicle-treated group with KA injection. The A. galanga extract showed the neuroprotective effect in KA-induced neurodegeneration. However, further studies are needed to explore the mechanisms of the extract to protect against neuronal loss.
References
World Health Organization [Internet]. Epilepsy; 2019 [cited 2019 Aug 11]. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
Bernasconi N. Is epilepsy a curable neurodegenerative disease? Brain. 2016;139(Pt 9):2336-7.
Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23(11):580-7.
Leite JP, Babb TL, Pretorius JK, Kuhlman PA, Yeoman KM, Mathern GW. Neuron loss, mossy fiber sprouting, and interictal spikes after intrahippocampal kainate in developing rats. Epilepsy Res. 1996;26(1):219-31.
Xie C, Sun J, Qiao W, Lu D, Wei L, Na M, et al. Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS One. 2011;6(9):e24966.
Gluck MR, Jayatilleke E, Shaw S, Rowan AJ, Haroutunian V. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res. 2000;39(1):63-71.
Nassiri-Asl M, Naserpour Farivar T, Abbasi E, Sadeghnia HR, Sheikhi M, Lotfizadeh M, et al. Effects of rutin on oxidative stress in mice with kainic acid-induced seizure. J Integr Med. 2013;11(5):337-42.
Milatovic D, Gupta RC, Dettbarn WD. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002;957(2):330-7.
Candelario-Jalil E, Al-Dalain SM, Castillo R, Martinez G, Fernandez OS. Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J Appl Toxicol. 2001;21(5):403-7.
Bloss EB, Hunter RG. Hippocampal kainate receptors. Vitam Horm. 2010;82:167-84.
Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14(9):5525-47.
Werner P, Voigt M, Keinanen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351(6329):742-4.
Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13(8):3582-98.
Ravizza T, Rizzi M, Perego C, Richichi C, Veliskova J, Moshe SL, et al. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia. 2005;46 Suppl 5:S113-7.
Zhang XM, Duan RS, Chen Z, Quezada HC, Mix E, Winblad B, et al. IL-18 deficiency aggravates kainic acid-induced hippocampal neurodegeneration in C57BL/6 mice due to an overcompensation by IL-12. Exp Neurol. 2007;205(1):64-73.
Martinc B, Grabnar I, Vovk T. Antioxidants as a preventive treatment for epileptic process: a review of the current status. Curr Neuropharmacol. 2014;12(6):527-50.
Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Watanabe H, Matsumoto K. Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci. 2006;78(16):1884-91.
Tadtong S, Watthanachaiyingcharoen R, Kamkaen N. Antimicrobial constituents and synergism effect of the essential oils from Cymbopogon citratus and Alpinia galanga. Nat Prod Commun. 2014;9(2):277-80.
Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100(3):237-43.
Tamura S, Shiomi A, Kimura T, Murakami N. Halogenated analogs of 1'-acetoxychavicol acetate, Rev-export inhibitor from Alpinia galanga, designed from mechanism of action. Bioorg Med Chem Lett. 2010;20(7):2082-5.
Seo JW, Cho SC, Park SJ, Lee EJ, Lee JH, Han SS, et al. 1'-Acetoxychavicol acetate isolated from Alpinia galanga ameliorates ovalbumin-induced asthma in mice. PLoS One. 2013;8(2):e56447.
Juntachote T, Berghofer E, Siebenhandl S, Bauer F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006;72(3):446-56.
Saha S, Banerjee S. Central nervous system stimulant actions of Alpinia galanga (L.) rhizome: a preliminary study. Indian J Exp Biol. 2013;51(10):828-32.
Hanish Singh JC, Alagarsamy V, Diwan PV, Sathesh Kumar S, Nisha JC, Narsimha Reddy Y. Neuroprotective effect of Alpinia galanga (L.) fractions on Abeta(25-35) induced amnesia in mice. J Ethnopharmacol. 2011;138(1):85-91.
Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5(3):515-28.
Berger ML, Charton G, Ben-Ari Y. Effect of seizures induced by intra-amygdaloid kainic acid on kainic acid binding sites in rat hippocampus and amygdala. J Neurochem. 1986;47(3):720-7.
Somerlik KH, Cosandier-Rimélé D, Cordeiro JG, Krüger TB. Measuring epileptogenicity in kainic acid injected rats. Proceedings of the 5th International IEEE EMBS Conference on Neural Engineering; 2011 April 27 - May 1; Cancun, Mexico, p. 188–91.
Levesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2887-99.
Ben-Ari Y, Lagowska J, Tremblay E, Le Gal La Salle G. A new model of focal status epilepticus: intra-amygdaloid application of kainic acid elicits repetitive secondarily generalized convulsive seizures. Brain Res. 1979;163(1):176-9.
Nadler JV. Kainic acid: neurophysiological and neurotoxic actions. Life Sci. 1979;24(4):289-99.
Vincent P, Mulle C. Kainate receptors in epilepsy and excitotoxicity. Neuroscience. 2009;158(1):309-23.
Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375-403.
Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392(6676):601-5.
Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH. Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia. 2004;45(10):1202-9.
Cheon S. Hippocampus-dependent Task Improves the Cognitive Function after Ovariectomy in Rats. Osong Public Health Res Perspect. 2017;8(3):227-34.
Groticke I, Hoffmann K, Loscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol. 2008;213(1):71-83.
Gobbo OL, O'Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125(2):317-27.
Maia GH, Quesado JL, Soares JI, do Carmo JM, Andrade PA, Andrade JP, et al. Loss of hippocampal neurons after kainate treatment correlates with behavioral deficits. PLoS One. 2014;9(1):e84722.
Matsuda H, Morikawa T, Managi H, Yoshikawa M. Antiallergic principles from Alpinia galanga: structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem Lett. 2003;13(19):3197-202.
Watanabe N, Kataoka T, Tajika T, Uramoto M, Magae J, Nagai K. 1′-Acetoxychavicol acetate as an inhibitor of phagocytosis of macrophages. Biosci Biotechnol Biochem. 1995;59(8):1566–7.
Yu ES, Min HJ, Lee K, Lee MS, Nam JW, Seo EK, et al. Anti-inflammatory activity of p-coumaryl alcohol-gamma-O-methyl ether is mediated through modulation of interferon-gamma production in Th cells. Br J Pharmacol. 2009;156(7):1107-14.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







