Ultrasound-assisted extraction of phenolic compounds from coconut endocarp and its radical scavenging activity
Keywords:
Coconut endocarp, Phenolic compounds, Radical scavenging activity, Ultrasound-assisted extraction (UAE)Abstract
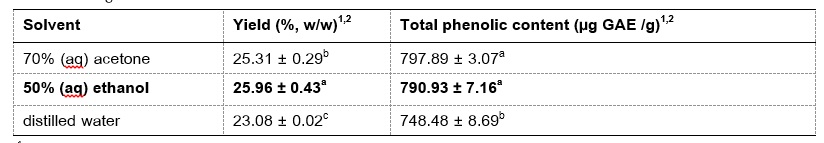
Phenolic compounds were extracted from the coconut endocarp waste, a by-product of the processing of coconut fruits. The optimum conditions for extraction of phenolic compounds from coconut endocarp using ultrasound-assisted extraction (UAE) were determined using three main parameters: solvent type, extraction temperature and extraction time. It was found that the extraction of sample with 50% (aq) of ethanol at 50 ºC for 120 minutes provided the highest crude extract yield (29.46% w/w on dry basis) and the highest total phenolic content (962.31 µg GAE/g). The antioxidant activity of crude ethanolic extract under the optimum condition was further investigated and the results showed that the crude extract exhibited strong antioxidant activity with IC50 values of 288.17 µg/ml by DPPH assay and 10.55 µg/ml by ABTS assay, respectively
References
Rodrigues S, Pinto GAS. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J Food Eng. 2007; 80: 869-872.
Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of northeast Portugal honey. Food Chem Toxicol. 2008; 46: 3774-3779.
Rodrigues S, Pinto GAS, Fernandes FAN. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason Sonochem. 2008; 15: 95-100.
Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement Altern Med. 2011; 11(130): 1-8.
Jabbar S, Abid M, Wu T, Hashim MM, Saeeduddin M, Hu B, et al. Ultrasound‐assisted extraction of bioactive compounds and antioxidants from carrot pomace: a response surface approach. J Food Process Preserv. 2015; 39(6): 1-11.
Bajalan I, Zand M, Goodarzi M, Darabi, M. Antioxidant activity and total phenolic and flavonoid content of the extract and chemical composition of the essential oil of Eremostachys laciniata collected from Zagros. Asian Pac J Trop Biomed. 2017; 7(2): 144-146.
Karagozler AA, Erdag B, Emek YC, Uygun DA. Antioxidant activity and proline content of leaf extracts from Dorystoechas hastata. Food Chem. 2008; 111: 400-407.
Murray JC, Burch JA, Streilein RD, Lannacchione MA, Hall RP, Pinnell SR. 2008. A tropical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008; 59: 418-425.
Vinatoru M, Toma M, Mason TJ. Ultrasonically assisted extraction of bioactive principles from plants and their constiturents. In: Mason TJ, editor. Adavances in Sonochemistry, vol. 5, Stamford, CT: JAI Press; 1999: 209-248.
Paniwnyk L, Beaufoy E, Lorimer JP, Mason TJ. The extraction of rutin from flower buds of Sophora japonica. Ultrason Sonochem. 2001; 8: 299-301.
Klen TJ, Vodopivec BM. Ultrasonic extraction of phenols from olive mill wastewater: comparison with conventional methods. J Agric Food Chem. 2011; 59: 12725-12731.
Rabelo RS, Machado MTC, Martínez J, Hubinger MD. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J Food Eng. 2016; 178: 170-180.
Al-Dhabi NA, Ponmurugan K, Maran P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason Sonochem. 2017; 34: 206-213.
Vu HT, Scarlett CJ, Vuong QV. Optimization of ultrasound‐assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J Food Process Pres. 2017; 41(5): 1-14.
Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999; 299: 152-178.
Siramon P, Ohtani Y. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. J Wood Sci. 2007; 53(6): 498-504.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26: 1231-1237.
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014; 22(3): 296–302.
BendiniA, Cerretani L, Pizzolante L, Toschi TG, Guzzo, F, Ceoldo S, et al. Eur. Food Res. Technol. 2005; 223(1): 102-109.
Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement. Altern. Med. 2011; 130(11): 1-8.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







