Comparative evaluation of antioxidant and anti-Inflammatory activities of four seaweed species from the east coast of the Gulf of Thailand
Keywords:
Macrophage, Nitric oxide, Phytochemicals, ROS; SeaweedAbstract
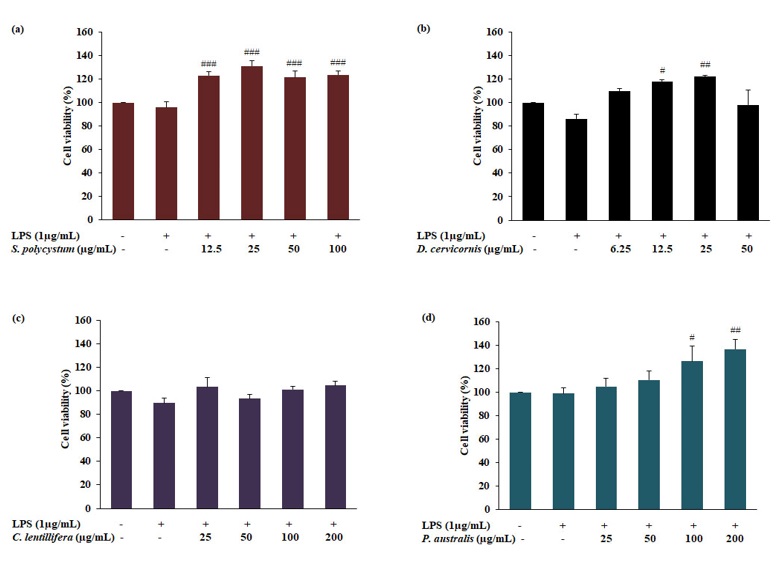
Seaweeds are good sources of bioactive secondary metabolites, with utilizations in medicine and the food industry. However, information on the biological activities of seaweed harvested from the east coast of the Gulf of Thailand are limited. We, therefore, conducted a comparatively study on antioxidant and anti-inflammatory activities of four seaweed species (Dictyota cervicornis, Sargassum polycystum, Padina australis and Caulerpa lentillifera) collected from Sattahip District, Chonburi Province. In vitro antioxidant screening was performed based on DPPH radical scavenging and metal ion chelating activities. Cell-based antioxidant activities were evaluated based on the formation of reactive oxygen species (ROS) by dichlorofluorescein (DCF) assay. Anti-inflammatory activity was determined by assessing suppression of nitric oxide (NO) production in LPS-induced macrophages. Among all seaweed species examined, P. australis was the most active as a DPPH scavenger, whereas P. australis and D. cervicornis showed the highest metal chelating activity. Furthermore, D. cervicornis showed the greatest inhibitory activity on ROS and NO production in LPS-stimulated macrophages. Phytochemical screening tests demonstrated the presence of steroids in all four seaweeds. Tannins were found in P. australis and D. Cervicornis and terpenoids in D. Cervicornis. These results suggest all four seaweeds, especially D. cervicornis, are natural sources of antioxidants and anti-inflammatory agents with potential applications in the food and medical industries.
References
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic Biol Med. 2010; 49(11): 1603-1616.
Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: Implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 2006; 15(3): 177-186.
de Araújo RFF, Martins DBG, Borba MA. Oxidative Stress and Disease. In: Morales-Gonzalez JA, Morales-Gonzalez A, Madrigal-Santillan EO, editors. A Master Regulator of Oxidative Stress-The Transcription Factor Nrf2. London: IntechOpen; 2016. DOI: 10.5772/65366.
Farvin KH, Surendraraj A, Al-Ghunaim A, Al-Yamani F. Chemical profile and antioxidant activities of 26 selected species of seaweeds from Kuwait coast. J Appl Phycol. 2019; 31: 2653-2668.
Ermakova S, Sokolova R, Kim SM, Um BH, Isakov V, Zvyagintseva T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl Biochem Biotechnol. 2011; 164(6):841-850.
Sharma BR, Rhyu DY. Anti-diabetic effects of Caulerpa lentillifera: stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian Pac J Trop Biomed. 2014; 4(7): 575-580.
Val A, Platas G, Basilio A, Cabello A, Gorrochategui J, Suay I, et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int Microbiol. 2001;
(1): 35-40.
Monsur HA, Jaswir I, Simsek S, Amid A, Alam Z, Tawakalit AH. Cytotoxicity and inhibition of nitric oxide syntheses in LPS induced macrophage by water soluble fractions of brown seaweed. Food Hydrocoll. 2014; 42: 269-274.
Shanura Fernando IP, Asanka Sanjeewa KK, Samarakoon KW, Lee WW, Kim HS, Ranasinghe P, et al. Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Sci Biotechnol. 2018; 27(6): 1761-1769.
Yang EJ, Moon JY, Kim MJ, Kim DS, Kim CS, Lee WJ, et L. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J Zhejiang Univ-SciB (Biomed&Biotechnol). 2010; 11(5): 315-322.
Yangthong M, Hutadilok-Towatana N, Phromkunthong W. Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Foods Hum Nutr.2009; 64(3): 218-223.
Peerapornpisal Y, Amornlerdpison D, Jamjai U, Taesotikul T, Pongpaibul Y, Nualchareon M, et al. Antioxidant and anti-inflammatory activities of brown marine alga, Padina minor Yamada. Chiang Mai J Sci. 2010; 37: 507-516.
Sumintilee W, Banjongsinsiri P, Praiboon J, Klaypradit W. Antioxidant activities of crude extracts from Caulerpa lintillifera, Sargassum oligocystum and Gracilaria changii. J Food Technol. 2014; 9(1): 63-75.
Saengkhae C, Jongaramruong J, Noiraksar T. Cytotoxic activities of crude extract from seaweeds along the Gulf of Thailand on cancer cells. Burapha Sci J. 2009; 14: 88-98.
Petchyothin P, Praiboon J, Chirapart A. Antibacterial activity of seaweed extracts against acne inducing bacteria (Propionibacterium acnes). KMUTT Res Dev J. 2015; 38(3): 273-282.
Srisook K, Buapool D, Boonbai R, Simmasut P, Charoensuk Y, Srisook E. Antioxidant and anti-inflammatory activities of hot water extract from Pluchea indica Less. herbal tea. J Med Plants Res. 2012; 6(23): 4077-4081.
Tongyen T, Flor L, Srisook E, Srisook K. Influence of extraction method on antioxidant and nitric oxide-stimulating activity of herbal mixtures in human endothelial cells. NU Int J Sci. 2018; 15(2): 58-66.
Srisook K, Srisook E, Nachaiyo W, Chan-In M, Thongbai J, Wongyoo K, et al. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J Ethnopharmacol. 2015; 165: 94-102.
Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, Ezennia, E, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008; 7(3):1019-1024.
Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010; 10(1): 21; doi: 10.1186/1472-6882-10-21.
Savithramma N, Rao ML, Suhrulatha D. Screening of medicinal plants for secondary metabolites. Middle East J Sci Res. 2011; 8(3): 579-584.
Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011; 3(12): 10-14.
Abramovič H, Grobin B, Poklar Ulrih N, Cigić B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and folin–ciocalteu. J Chem. 2018; 2018: Article ID 4608405.
Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress. Eur J Med Chem. 2015; 97: 55-74.
Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2010; 10: 128-134.
Nursid M, Marasskuranto E, Atmojo KB, Hartono MP, Meinita MDN, Riyanti R. Investigation on antioxidant compounds from marine algae extracts collected from Binuangeun Coast, Banten, Indonesia. Squalen Bull of Mar and Fish Postharvest and Biotec. 2016; 11(2): 59-67.
Cox S, Abu-Ghannam N, Gupta S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int Food Res J. 2010; 17: 205-220.
Lima RL, Pires-Cavalcante KM, Alencar DB, Viana FA, Sampaio AH, Saker-Sampaio S. In vitro evaluation of antioxidant activity of methanolic extracts obtained from seaweeds endemic to the coast of Ceará, Brazil. Acta Sci Technol. 2016; 38(2): 247-255.
Wen ZS, Xiang XW, Jin HX, Guo XY, Liu LJ, Huang YN, et al. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264. 7 macrophages. Int J Biol Macromol. 2016; 88: 403-413.
Chen J, Li H, Zhao Z, Xia X, Li B, Zhang J, et al. Diterpenes from the marine algae of the genus Dictyota. Marine Drugs 2018; 16(5): 159; doi:10.3390/md16050159.
Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem. 2009; 57(9): 3483-3489.
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013; 138(2-3): 1764-1785.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







