Production of Vitamin D Enriched Yeast with UV-B Irradiation and the Degradation of Vitamin D in Rumen Fluid of Thai Native Cattle
Keywords:
Vitamin D2, Vitamin D3, UV-B irradiated vitamin D enrich yeastAbstract
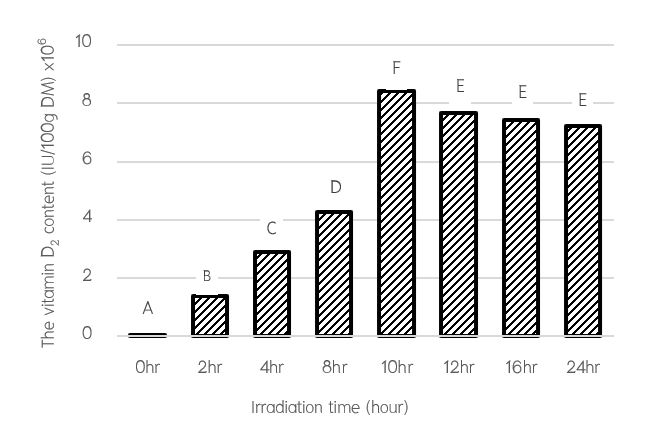
The purposes of this study were to investigate the effect of UV-B exposure condition to produce vitamin D enrich yeast and analyze degradation of UV-B irradiated vitamin D enrich yeast in the rumen fluid from Thai native cattle. The baker’s yeast (Saccharomyces cerevisiae) was selected from single colony of instant active dry yeast by streak plate method for the investigation of the optimum UV-B irradiation time. Yeasts were exposed to different UV-B irradiation for 0, 2, 4, 8, 10, 12, 16 and 24 hours. This study found that UV-B irradiation of all treated yeast enhanced the amount of vitamin D2 and the maximum amount of vitamin D2 at 10 hours (70.5 to 8409277.11 IU/100g DM) was significantly different (p <0.05). The rumen fluid from 4 fistulated Thai native cattle were used for analyze degradation of UV-B irradiated vitamin D enrich yeast. The treatments were divided into 5 groups, which were rumen liquor buffer without vitamin D and yeast supplementation (Control) and those with non-irradiation yeast supplementation (Non UV-B IY), vitamin D2 (VIT D2), vitamin D3 (VIT D3) and UV-B irradiated vitamin D enrich yeast (UV-B IVDRY). The samples were taken after overnight incubation at 39 °C, shaking at 70 rpm for 24 hours in order to analyze of degradation of vitamin D after incubation in the rumen. This study was found that the VIT D2 and VIT D3 groups had decreased vitamin D content from the initial supplementation (411.56 μg compared to 205.48 μg and 393.48 μg compared to 178.44 μg or decreased about 50.07% and 54.64%, respectively). On the other hand, the UV-B IVDRY group had decreased amount of vitamin D at only 18.22 μg or 4.47%. The amount of vitamin D was decreased less than the VIT D2 and VIT D3 groups (p <0.05). Therefore, supplementing UV-B irradiated vitamin D enrich yeast as a source of vitamin D to dairy cows can help to decrease the loss of vitamin D that is degraded by the rumen microbes, allowing to be more absorbed and utilized.
References
A. O. A. C. Official methods of analysis. 18thedn. Gaithersburg, MD, USA: Association of Official Analytical Chemists; 2000.
Abe F, Hiraki T. Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae. J Biochimica et Biophysica Acta. 2009; 1788(3):743-752.
Arnold WN. The structure of the yeast cell wall solubilization of a marker enzyme, β-fructofuranosidase, by the autolytic enzyme system. J Biological Chemistry. 1972; 247(4):1161-1169.
Braun M, Fuss W, Kompa KL. Improved photosynthesis of previtamin D by wavelengths of 280-300 nm. J Photochem Photobio. 1991; 61:15-26.
Dias ALG, Freitas JA, Micai B, Azevedo RA, Greco LF, Santos JEP. Effect of supplement yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. . J Dairy Sci. 2017; 101:201-221.
Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005; 289:8-28.
Elena M, Agafia U, Nadejda E, Natalia C, Ludmila F. Biotechnological aspects concerning the ergosterol obtianing from yeasts. J Analele University din Oradea, Fascicula Biologie. 2013; 1:12-18.
Foss YJ. Vitamin D deficiency is the cause of common obesity. J Medical Hypotheses. 2009; 72(3):314–321.
Jasinghe VJ, Perera CO. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. J Food Chem. 2005; 92(3):541-546.
Jouany JP. Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. J Anim. Reprod. Sci. 2006; 96: 250-264.
Jouany JP, Mathieu F, Senaud J, Bohatier J, Bertin G, Mercier M. Effects of Saccharomyces cerevisiae and Aspergillus oryzae on the population of rumen microbes and their polysaccharidase activities. South African J Anim. Sci. 1999; 29:63-64.
Mattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE. Vitamin D contents in edible mushrooms. J Agric Food Chem. 1994; 42:2449-2453.
Menke KH, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Food Chem. 1979; 92:217-222.
Reiner S, Micolod D, Schneiter R. Saccharomyces cerevisiae a model to study sterol uptake and transport in eukaryotes. J Biochemical Society Transactions. 2005; 33:1186-1188.
Schmid A, Walther B. Natural vitamin D content in animal products. J Adv Nutr. 2013; 4:453-462.
Sommerfeldt JL, Horst RL, Littledike ET, Beitz DC. In vitro degradation of cholecalciferol in rumen fluid. J Dairy Sci. 1979; 62(1):192–193.
Sommerfeldt JL, Horst RL, Napoli JL, Beitz DC, Littledike ET. Evidence for in vitro production of vitamin D2 and vitamin D3 metabolites by rumen microbes. J Dairy Sci. 1980; 63:88-92.
Steel RGD, Torrie JH. Principles and Procedures of statistics. New York: McGraw-Hill Book Co; 1960.
Teichmann R, Dutta PC, Staffas A, Jagerstad M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. J LWT - Food Science and Technology. 2007; 40(5):815–822.
Walker GM. Yeast Physiology and Biotechnology. England: John Wiley and Sons Ltd; 1998.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







