Hippocampal proteomic changes in a rat model of depression induced by dexamethasone
Keywords:
Proteomic analysis, Depression, Hippocampus, Dexamethasone, Synaptic dysfunctionAbstract
This study aimed to examine possible alterations of protein expression in the hippocampus of a rat model of depression induced by dexamethasone using the proteomic technique. The altered expression of several proteins has been found in a depressive group. The identified proteins play an important role in the cell signalling process, consisting of serotonergic, norepinephrinergic, dopaminergic, glutamatergic, and GABAergic receptors, the synaptic signalling associated proteins and the protein markers of GABAergic system. These results indicate the abnormality of signal transduction processes and the neurotransmitter dysfunction in depression. Moreover, up-regulation of beta-nerve growth factor, a neurotrophic factor involved in the survival of neurons, and down-regulation of amyloid-beta A4 precursor protein-binding family A member 1, a protein involved in the processing of the amyloid-beta precursor protein and signal transduction processes, were found in the depressive group. These findings reveal that the identified proteins in the hippocampus of the depressive model are important in the synaptic transmission process. It also shows synaptic dysfunction in depression. In summary, the identified proteins in this study by the proteomics technique could be used as the protein markers for further investigation.
References
Krishnan V, Nestler E J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902.
Martins-de-Souza D, Harris LW, Guest PC, Turck CW, Bahn S. The role of proteomics in depression research. European archives of psychiatry and clinical neuroscience. 2010;260(6):499–506.
Harrison PJ. The neuropathology of primary mood disorder. Brain : a journal of neurology. 2002;125(Pt 7):1428–1449.
Mu J, Xie P, Yang ZS, Yang DL, Lv FJ, Luo TY, et al. Neurogenesis and major depression: implications from proteomic analyses of hippocampal proteins in a rat depression model. Neuroscience letters. 2007;416(3): 252–256.
Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367(1601):2485–2494.
Sarchiapone M, Carli V, Camardese G, Cuomo C, Di Giuda D, Calcagni ML, et al. Dopamine transporter binding in depressed patients with anhedonia. Psychiatry research. 2006;147(2-3):243–248.
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European journal of pharmacology. 1990;185(1):1–10.
Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological psychiatry. 2005;58(4):297–306.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(1):70–75.
Petty F, Schlesser MA. Plasma GABA in affective illness. A preliminary investigation. Journal of affective disorders. 1981;3(4):339–343.
Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. The American journal of psychiatry. 1981;138(8):1098–1101.
Honig A, Bartlett JR, Bouras N, Bridges PK. Amino acid levels in depression: a preliminary investigation. Journal of psychiatric research. 1988;22(3):159–164.
Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(6):1050–1062.
Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Annals of the New York Academy of Sciences. 2009;1179:144–152.
Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment?. Psychoneuroendocrinology. 2011;36(3):415–425.
Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of psychiatry & neuroscience : JPN. 2004;29(6):417–426.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological psychiatry. 2000;48(8):755–765.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493.
Casarotto PC, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17(11):735–742.
Sigwalt AR, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011;192:661–674.
Skupio U, Tertil M, Sikora M, Golda S, Wawrzczak-Bargiela A, Przewlocki R. Behavioral and molecular alterations in mice resulting from chronic treatment with dexamethasone: relevance to depression. Neuroscience. 2015;286:141–150.
Danilczuk Z, Sekita-Krzak J, Lupina T, Danilczuk M, Czerny K. Influence of dizocilpine (MK-801) on neurotoxic effect of dexamethasone: behavioral and histological studies. Acta neurobiologiae experimentalis. 2006; 66(3):215–226.
Li C, Guo Z, Zhao R, Sun W, Xie M. Proteomic Analysis of Liver Proteins in a Rat Model of Chronic Restraint Stress-Induced Depression. BioMed Research International. 2017;2017: Article ID 7508316.
Comes AL, Papiol S, Müller T, Geyer PE, Mann M, Schulze T. Proteomics for blood biomarker exploration of severe mental illness: pitfalls of the past and potential for the future. Translational Psychiatry. 2018;8:160.
Reig-Viader R, Sindreu C, Bayés À. Synaptic proteomics as a means to identify the molecular basis of mental illness: Are we getting there?. Progress in neuro-psychopharmacology & biological psychiatry. 2018;84(Pt B):353–361.
Bosch PJ, Peng L, Kivell BM. Proteomics Analysis of Dorsal Striatum Reveals Changes in Synaptosomal Proteins following Methamphetamine Self-Administration in Rats. PloS one. 2015;10(10):e0139829.
Johansson C, Samskog J, Sundström L, Wadensten H, Björkesten L, Flensburg J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics. 2006;6(16):4475–4485.
Thorsell A, Portelius E, Blennow K, Westman-Brinkmalm A. Evaluation of sample fractionation using micro-scale liquid-phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid communications in mass spectrometry : RCM. 2007;21(5):771–778.
Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20(18):3551–3567.
Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, et al. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic acids research. 2017;45(D1):D183–D189.
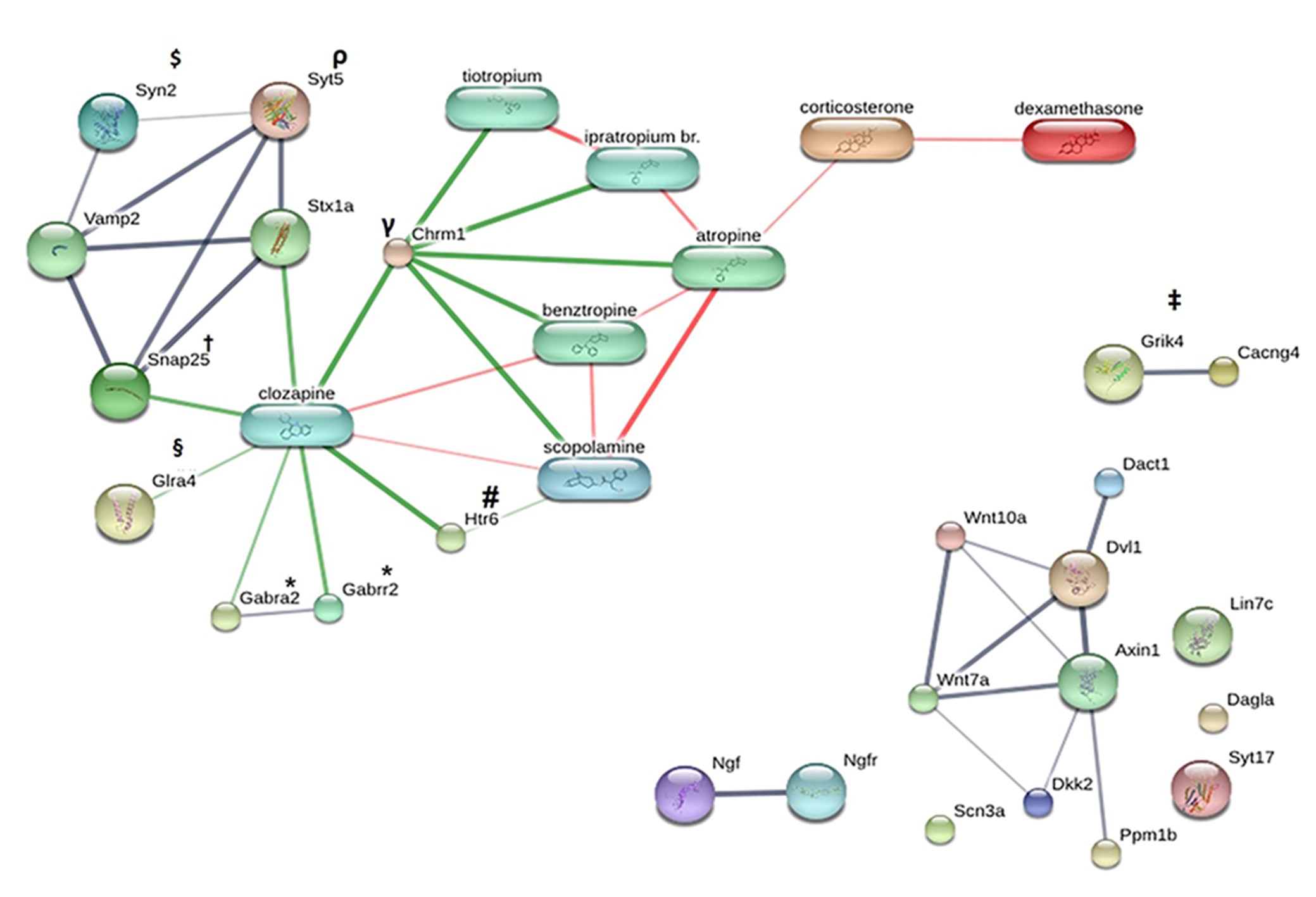
Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic acids research. 2016;44(D1):D380–D384.
Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12(15):3257–3262.
Balkarli A, Sengül C, Tepeli E, Balkarli H, Cobankara V. Synaptosomal-associated protein 25 (Snap-25) gene polymorphism frequency in fibromyalgia syndrome and relationship with clinical symptoms. BMC musculoskeletal disorders. 2014;15:191.
Ferreira A, Han HQ, Greengard P, Kosik KS. Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9225–9229.
Baker K, Gordon SL, Grozeva D, van Kogelenberg M, Roberts NY, Pike M, et al. Identification of a human synaptotagmin-1 mutation that perturbs synaptic vesicle cycling. The Journal of clinical investigation. 2015;125(4):1670–1678.
Awasthi A, Ramachandran B, Ahmed S, Benito E, Shinoda Y, Nitzan N, et al. Synaptotagmin-3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science (New York, N.Y.). 2019;363(6422):eaav1483.
Escribá PV, Ozaita A, García-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(8):1512–1521.
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. The American journal of psychiatry. 2011;168(7):727–734.
Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(6):1478–1485.
Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Molecular psychiatry. 2009;14(2):175–189.
Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotoxicity research. 2004;6(1):57–61.
Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. Journal of neural transmission (Vienna, Austria : 1996). 2007;114(7):893–898.
Miller CC, McLoughlin DM, Lau KF, Tennant ME, Rogelj B. The X11 proteins, Abeta production and Alzheimer's disease. Trends in neurosciences. 2006;29(5):280–285.
Yun HM, Park KR, Kim EC, Kim S, Hong JT. Serotonin 6 receptor controls Alzheimer's disease and depression. Oncotarget. 2015;6(29):26716–26728.
de Azevedo Cardoso T, Mondin TC, Wiener CD, Marques MB, Fucolo B, Pinheiro RT, et al. Neurotrophic factors, clinical features and gender differences in depression. Neurochemical research. 2014;39(8):1571–1578.
Liu X, Zhang T, He S, Hong B, Peng D, Su H, et al. Nerve growth factor variations in patients with mood disorders: no changes in eight weeks of clinical treatment. Neuropsychiatric disease and treatment. 2014;10:835–840.
Diniz BS, Teixeira AL, Machado-Vieira R, Talib LL., Gattaz WF, Forlenza OV. Reduced serum nerve growth factor in patients with late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21(5):493–496.
Martino M, Rocchi G, Escelsior A, Contini P, Colicchio S, de Berardis D, et al. NGF serum levels variations in major depressed patients receiving duloxetine. Psychoneuroendocrinology. 2013;38(9):1824–1828.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







