The biological activities of Kombucha during Fermentation Process
Keywords:
Kombucha, Yeast, Acetic acid bacteria, Antioxidant activity, Antibacterial activityAbstract
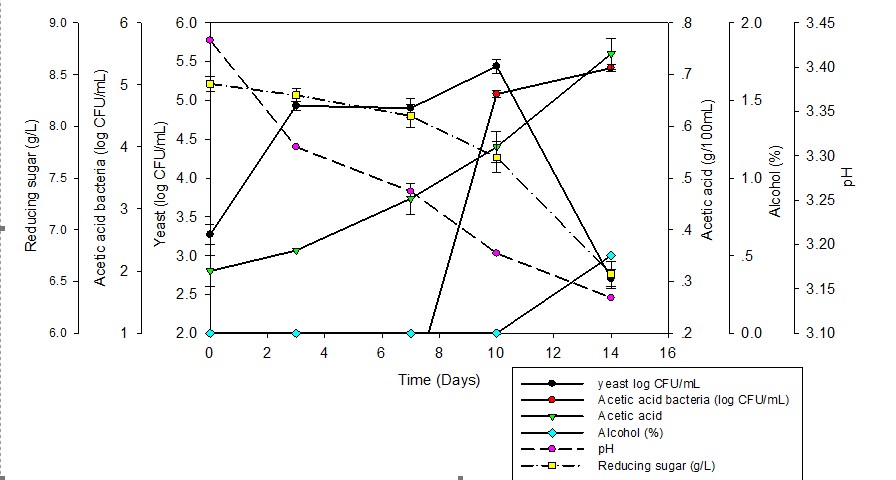
Aim of this research is to evaluate the biological activities of Kombucha during fermentation process and determined potential benefits of kombucha product, including the viable counts of acetic acid bacteria and yeasts, antioxidant activity, total phenolic contents and antibacterial activity were investigated. The brewing kombucha for 14 days was studied. The results show that yeast cells drastically increased withthin 10 days lead to the growth of 5.40±0.09 log CFU/mL while acetic acid bacteria increased of 5.27±0.05 log CFU/mL withthin 14 days was observed. According to acetic acid production has increased to 0.74±0.03 g/100 mL and reduces the pH at 3.14. The ethanol content of the kombucha was 0.5 % by volume and reducing sugar of 6.57±0.01 g/L was found. Antibacterial activity of kombucha in during fermentation process was determined which against Bacillus subtilis, B. cereus, Salmonella sp., Stayphylococcus aureus and Escherichia coli by agar well diffusion method. According to the antibacterial activity of 14 days kombucha brewing, have shown an ability to inhibit the growth of bacteria and also has antimicrobial activity against a spectrum of organism. The maximum activity was founded at 14 days of kombucha fermentation organism against all isolates, the AI value was 0.93±0.10, 1.49±0.17, 1.30±0.10, 0.98±0.10 and 1.12±0.24, respectively. The antioxidant activity was determined using the DPPH and ABTS methods. Kombucha samples exhibited increasing antioxidant activity during in the fermentation process. The 14 days of kombucha sample showed highest antioxidant activity by inhibiting 57.31±0.66 % of DPPH (equivalent to ascorbic acid 254.99 µg/mL) and showed 15.66±0.15 mM Trolox Equivalent Antioxidative Capacity for ABTS assay. Remarkable high phenolic content was found in 14 days kombucha brewing 0.99±0.04 mg gallic acid equivalents per milliliter. The result of present study revealed that kombucha could be the potential health benefit as antioxidant and antimicrobial activities which were increased with time of fermentation and demonstrated a higher activity compared to black tea.
References
Vajragupta O, Boonchoong P, Boonyarat C and Audsintong M. Radical scavenging agents. Bangkok: SP Print. 2549; 123-44.
Dufresne C, Farnworth E. Tea, Kombucha, and health: a review. Food Res. Int. 2000; 33(6): 409-421.
Yang Z, Ji B., Zhou F, Li B, Luo Y, Yang L, et al. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J. Sci. Food Agric. 2009; 89(1): 150-156.
Wang K, Gan X, Tang X, Wang S, Tan H. Determination of DSaccharic acid -1, 4 –lactone from brewed kombucha broth by high performance capillary electrophoresis. J. Chromatogr. 2010; 878: 371-374.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959; 31: 426-428.
A.O.A.C. Official Method of Analysis. The Association of Official Analytical Chemists. 2000. Gaithersburg, Maryland.
Lu M, Yuan B, Zeng M and Chen J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Research International. 2011; 44: 530- 536.
Wiriyaphan C, Chitsomboon B, Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012; 132: 104–111.
Matthäus B. Antioxidant Activity of Extracts Obtained from Residues of Different Oilseeds. J. Agric. Food Chem. 2002; 50: 3444–3452.
Mayo WJ. Chemical Methods of Control: Antimicrobial Drugs. In: Laboratory Experiments in Microbiology. Johnson TR, Case C.L. (Eds.), The Benjamin /Cummings PubLishing Company, San Francisco, CA, USA. 1998. pp. 179–181.
Assatarakul K, Himasuttidach N. Antioxidant and Antibacterial Activities of Onion Extract and Applications in Mixed Fruit and Vegetable Juice. Journal of Food Technology, Siam University. 2017; 12(1)1; 71-83.
Bacteriological Analytical Manual Online. Chapter 12: Staphylococcus aureus. USFDA. 2006; 10 pp. (http://www.cfsan.fda.gov).
Bacteriological Analytical Manual Online. Chapter 5: Salmonella. USFDA. 2007; 10 pp. (http://www.cfsan.fda.gov).
Bacteriological Analytical Manual Online. Chapter 4: Enumeration of Escherichia coli and thecoliform bacteria. USFDA. 2002; 10 pp. (http://www.cfsan.fda.gov).
Bacteriological Analytical Manual Online. Chapter 14: Bacillus cereus. USFDA. 2012; 10 pp. (http://www.cfsan.fda.gov).
Reiss J. Influence of different sugars on the metabolism of the tea fungus. Zeitschrift für Lebensmittel- Untersuchung und -Forschung A.1994; 198: 258-261.
Yang Z, Ji B, Zhou F, Li B, Luo Y, Yang L and Li T. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J. Sci. Food Agric. 2009; 89 (1); 150-156.
Radomir V Malbaša Eva S Lončar Jasmina S.VitasJasna M Čanadanović-Brunet. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chemistry. 2011; 127: 1727–1731.
Jayabalan R, Marimuthu S and Swaminathan K. Changes in content of organic acids and tea polyphenols during Kombucha tea fermentation. Food Chem. 2007; 102: 392–398.
Charoenrak S, Bovonsombut S, Bovonsombut S. Isolation of Acetic Acid Bacteria for Kombucha Production by Pure Culture. Proceedings The 8th Science Research Conference. 30-31 May 2016. University of Phayao.
Amarasinghe H, Weerakkody NS, Waisundara VY. Evaluation of physicochemical properties and antioxidant activities of kombucha “Tea Fungus” during extended periods of fermentation. Food Sci Nutr. 2018; May 6(3): 659–665.
Chakravorty S, Bhattacharya S, Chatzinotas A, Chakraborty W, Bhattacharya D, Gachhui R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016; 220: 63–72.
Ivanišová E, Meňhartová K, Terentjeva M, Godočíková L, Árvay J, and Kačániová M. Kombucha tea beverage: Microbiological characteristic, antioxidant activity, and phytochemical composition. Acta Alimentaria. 2019; 48(3): 324–331.
Chu S C, Chen C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006; 98: 502–507.
Dibner J J, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002; 11: 453–463.
Ludovico P, Sansonetty F, Silva MT, Corte-Real M. Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEMS Microbiol. Lett. 2003; 3: 91–96.
Greenwalt C, Ledford R, Steinkraus K. Determination and characterization of the antimicrobial activity of the fermented tea Kombucha. LWT - Journal of Food Science and Technology. 1998; 31: 291–296.
Sreeramulu G, Zhu Y, Knol W. Kombucha Fermentation and Its Antimicrobial Activity. J. Agric. Food Chem. 2000; 48(6): 2589–2594.
Kaewkod T, Bovonsombut S, Tragoolpua Y. Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms. 2019; 7(12): 1-18.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







