Proteomic analysis of frontal cortex proteins in a rat model of depression induced by dexamethasone
Keywords:
Proteomics, Depression, Frontal cortex, DexamethasoneAbstract
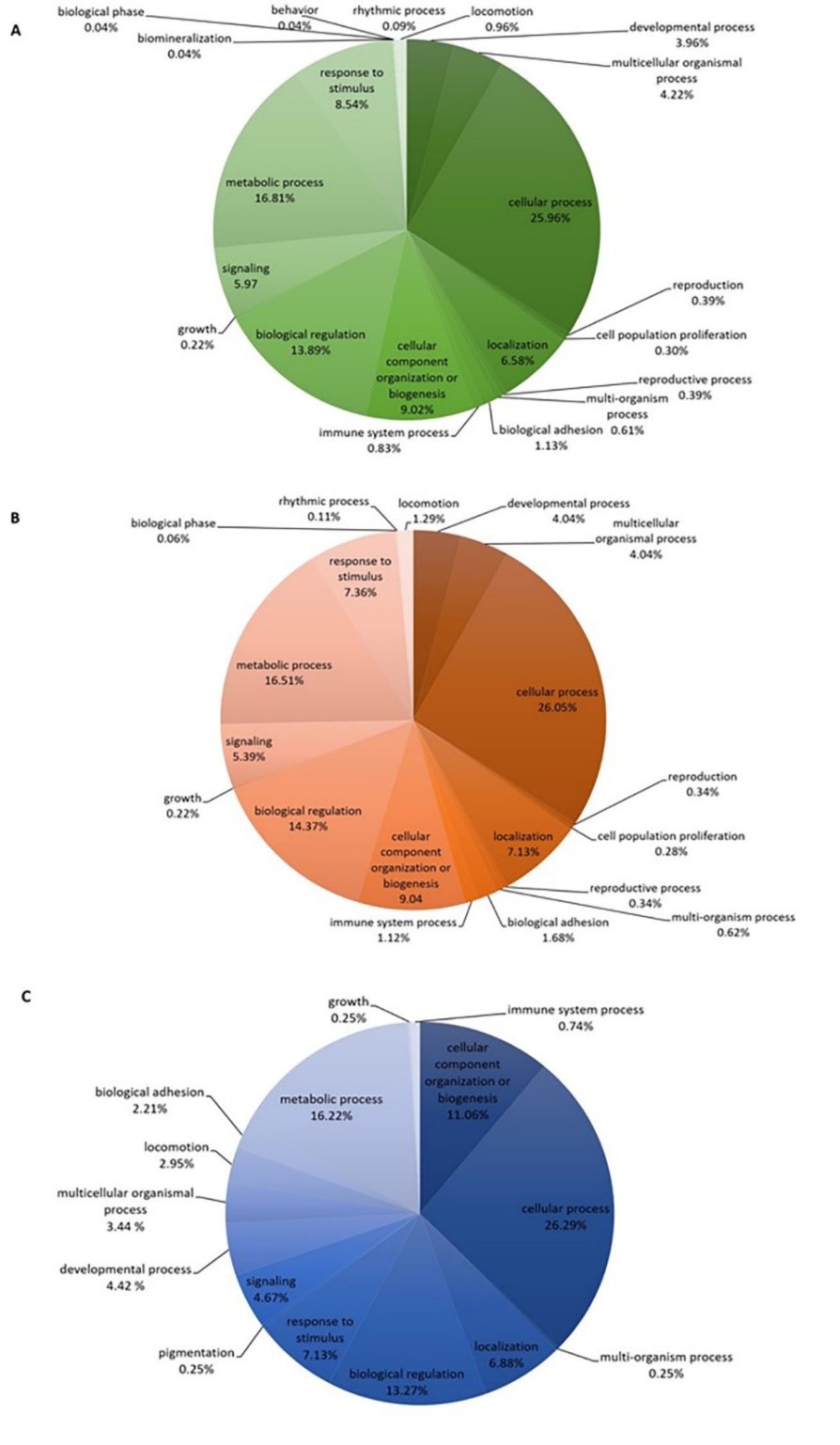
This study was designed to characterize the identified proteins in the frontal cortex of a rat model of depression induced by dexamethasone using proteomic technique. Identified proteins were analyzed according to the biological function. The identified proteins were categorized in the response to the cellular process, metabolic process, and others including cell-cell signaling, which are associated with the brain functions. Results showed that uniquely expressed proteins in control and depressive groups reveal the function of monoamine, glutamate and GABA receptors and appear to be a part of the protein transporter, the regulating protein of cell communication and synapse. In addition, a decrease of the co-expressed proteins was found in the depressive group when compared to the control group. These proteins are associated with the function of the dopamine neurotransmitter system. The results of this study suggest that the associated proteins in synaptic transmission, particularly neurotransmitter receptors, regulating proteins of cell communication and synapse play an important role in the pathophysiology of depression. Furthermore, the identified proteins in this study may be used as a biological marker in the study of frontal cortex function to further clarify the mechanism for depression. That may lead to appropriate treatment.
References
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455 (7215):894-902.
Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367 (1601): 2485-94.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383-406.
Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric disease and treatment. 2011;7(Suppl 1):9.
Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415-25.
Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1601):2485-94.
Placidi GP, Oquendo MA, Malone KM, Huang Y-Y, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biological psychiatry. 2001;50(10):783-91.
Sarchiapone M, Carli V, Camardese G, Cuomo C, Di Giuda D, Calcagni M-L, et al. Dopamine transporter binding in depressed patients with anhedonia. Psychiatry Research: Neuroimaging. 2006;147(2-3):243-8.
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European journal of pharmacology. 1990;185(1):1-10.
Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KRR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological Psychiatry. 2005;58(4): 297-306.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(1):70-5.
Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA B receptors in the modulation of anxiety-and antidepressant-like behavior. Neuropsychopharmacology. 2004;29(6):1050-62.
Petty F, Schlesser MA. Plasma GABA in affective illness: A preliminary investigation. Journal of affective disorders. 1981;3(4):339-43.
Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. The American journal of psychiatry. 1981.
Honig A, Bartlett J, Bouras N, Bridges P. Amino acid levels in depression: a preliminary investigation. Journal of Psychiatric Research. 1988;22(3):159-64.
Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain research reviews. 2008;57(2):561-70.
Pariante CM. Risk factors for development of depression and psychosis: glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Annals of the New York Academy of Sciences. 2009;1179:144.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological psychiatry. 2000;48(8):755-65.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. Neurogenesis-dependent and-independent effects of fluoxetine in an animal model of anxiety/ depression. Neuron. 2009;62(4):479-93.
Casarotto P, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. European Neuropsychopharmacology. 2007;17 (11):735-42.
Sigwalt A, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011;192:661-74.
Skupio U, Tertil M, Sikora M, Golda S, Wawrzczak-Bargiela A, Przewlocki R. Behavioral and molecular alterations in mice resulting from chronic treatment with dexamethasone: relevance to depression. Neuroscience. 2015;286:141-50.
Uys JD, Stein DJ, Daniels WM. Neuroproteomics: relevance to anxiety disorders. Current Psychiatry Reports. 2006;8(4):286-90.
Martins-de-Souza D, Harris LW, Guest PC, Turck CW, Bahn S. The role of proteomics in depression research. European archives of psychiatry and clinical neuroscience. 2010;260(6):499-506.
Bosch, P. J., Peng, L., & Kivell, B. M. Proteomics Analysis of Dorsal Striatum Reveals Changes in Synaptosomal Proteins following Methamphetamine Self-Administration in Rats. PloS one, 2015;10(10), e0139829.
Sharma A, Roytrakul S, Kittisenachai S, Semprasert N, and Kooptiwut S. Alteration in pancreatic protein expression in dexamethasone-treated mice. Songklanakarin J Sci Technol. 2020;42(3): 477-486.
Johansson C, Samskog J, Sundström L, Wadensten H, Björkesten L, Flensburg J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC‐MS/MS data. Proteomics. 2006;6(16):4475-85.
Thorsell A, Portelius E, Blennow K, Westman‐Brinkmalm A. Evaluation of sample fractionation using micro‐scale liquid‐phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass Spectrometry. 2007;21(5):771-8.
Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability‐based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis: An International Journal. 1999;20(18):3551-67.
Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, et al. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic acids research. 2017;45(D1):D183-D9.
Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic acids research. 2016;44(D1):D380-D4.
Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12(15): 3257-62.
Hettema J, An S, Neale M, Bukszar J, Van den Oord E, Kendler K, et al. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006;11(8):752-62.
Veerasakul S, Watiktinkorn P, Thanoi S, Reynolds GP, Nudmamud-Thanoi S. Association of polymorphisms in GAD1 and GAD2 genes with methamphetamine dependence. Pharmacogenomics. 2017;18(1): 17-22.
Escribá PV, Ozaita A, García-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29 (8):1512-21.
Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Molecular brain. 2017;10(1):1-12.
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [11C] ABP688 PET and postmortem study. American Journal of Psychiatry. 2011;168 (7):727-34.
Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Current behavioral neuroscience reports. 2015;2(3): 146-53.
Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Progress in neuro-psychopharmacology & biological psychiatry. 2002.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. science. 2007;318(5847):71-6.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







