Proteomics analysis of rat testis reveals changes of proteins involving the signal transduction after methamphetamine exposure
Keywords:
Methamphetamine, Proteomics, Signal transduction, Testis, RatAbstract
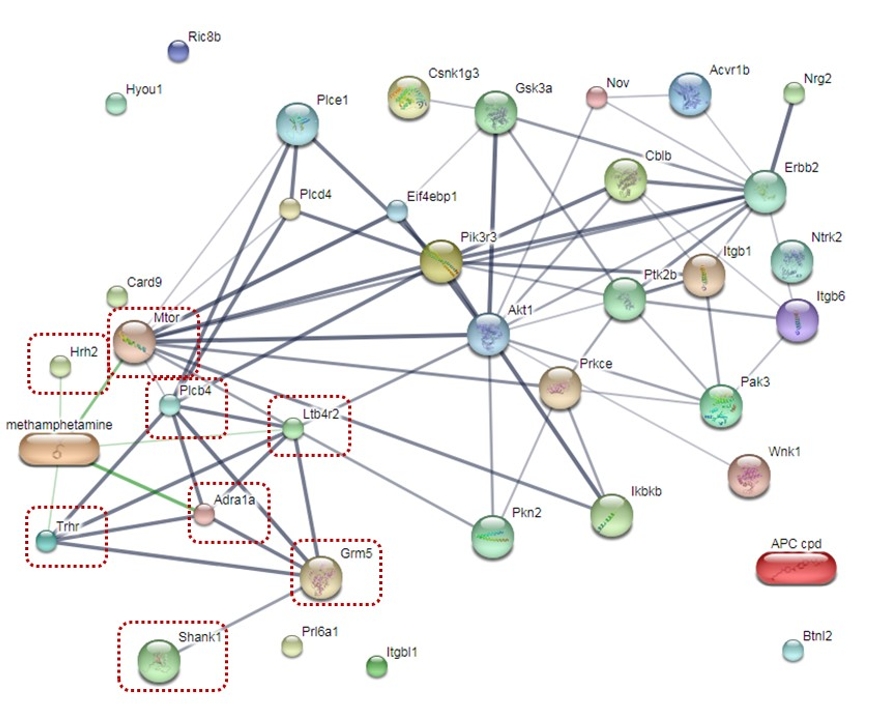
Methamphetamine (METH) is an addictive drug potentially affecting the male reproductive system. It causes poor sperm quality and an increase in apoptotic cells within the seminiferous tubule. METH administration can also result in the changes of dopamine, norepinephrine, and GABA in rat testis. These findings provide the hypothesis that METH might associate with the changes of proteins involving biological process in spermatogenesis. Therefore, the aim of this study is to investigate the expression of the signal transduction proteins underlying biological processes in the testis of METH-administered rats using proteomics analysis. Male Sprague-Dawley rats in a control group were received normal saline for 15 days, whereas those rats in an ED-binge METH group were received an escalating dose of METH for 14 days following a binge dose of METH on day 15. Proteins were extracted from the rat testis, which were pooled from three samples in each group. The liquid chromatography-tandem mass spectrometry was performed to identify the protein profiles. The total of 383 proteins were identified in the testis of both groups. 38 proteins were mapped in the signal transduction sub-class underlying biological processes including 19 proteins with up-regulated expression and 19 proteins with down-regulated expression. Moreover, there were 8 proteins responding to METH exposure. In conclusion, our findings will be useful for better understanding of METH on protein expression resulting in the impairment of spermatogenesis in the testis.
References
Ziedonis D, Williams JM and Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. The American Journal of Med Science 2003; 326(4):223-230.
Zweben JE, Cohen JB., Christian D, Galloway GP, Salinardi M, Parent D and Iguchi M. Psychiatric symptoms in methamphetamine users. The American Journal on addiction 2004; 13(2):181-190.
Sabol KE, Roach JT, Broom SL, Ferreira C, Preau MM. Long-term Effects of a High-Dose Methamphetamine Regimen on Subsequent Methamphetamine-Induced Dopamine Release in Vivo. Brain Research 2001; 892(1):122-129.
Ferrucci M, Limanaqi F, Ryskalin L, Biagioni F, Busceti CL and Fornai F. The Effects of Amphetamine and Methamphetamine on the Release of Norepinephrine, Dopamine and Acetylcholine from the Brainstem Reticular Formation. Frontiers in neuroanatomy 2019; 13:48.
Nudmamud-Thanoi S, Veerasakul S and Thanoi S. Pharmacogenetics of drug dependence: Polymorphisms of genes involved in GABA neurotransmission. Neuroscience Letters 2019; 134128.
Nudmamud-Thanoi S, Iamjan SA, Kerdsan-Phusan W, Thanoi S. Pharmacogenetics of drug dependence: Polymorphisms of genes involved in glutamate neurotransmission. Neuroscience Letters 2019; 134128.
Dickerson SM, Walker DM, Reveron ME, Duvauchelle CL and Gore AC. The recreational drug ecstasy disrupts the hypothalamic-pituitary-gonadal reproductive axis in adult male rats. Neuroendocrinology 2008; 88(2):95-102.
Yamamoto Y, Yamamoto K, Hayase T, Abiru, H, Shiota K and Mori C. Methamphetamine induces apoptosis in seminiferous tubules in male mice testis. Toxicology and Applied Phamacology 2002; 178(3):155–160.
Alavi SH, Taghavi MM, Moallem SA. Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. System Biology in Reproductive Medicine 2008; 54(2):85–91.
Nudmamud-Thanoi S and Thanoi S. Methamphetamine induces abnormal sperm morphology, low sperm concentration and apoptosis in the testis of male rats. Andrologia 2011; 43(4):278-282.
Nudmamud-Thanoi S, Sueudom W, Tangsrisakda N and Thanoi S. Changes of sperm quality and hormone receptors in the rat testis after exposure to methamphetamine. Drug and Chemical Toxicology 2016; 39(4): 432–438.
Janphet S, Nudmamud-Thanoi S and Thanoi S. Alteration of catecholamine concentrations in rat testis after methamphetamine exposure. Andrologia 2016; 49(2): e12616.
Kaewman P, Nudmamud-Thanoi S and Thanoi S. GABAergic Alterations in the Rat Testis after Methamphetamine Exposure. International Journal of Medical Sciences 2018; 15(12): 1349–1354.
Cannarella R, Condorelli RA, Mongioì LM, Vignera SL and Calogero AE. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. International Journal of Molecular Science 2020; 21(5):1728.
Huang XY and Sha JH. Proteomics of spermatogenesis: from protein lists to understanding the regulation of male fertility and infertility. Asian Journal Andrology 2011; 13(1):18–23.
Mallick P and Kuste B. Proteomics: a pragmatic perspective. Nature Biotechnology 2010; 28:695–709.
Aslam B, Basit M, Nisar MA, Khurshid M and Rasool MH. Proteomics: Technologies and Their Applications. Journal of Chromatographic Science 2017; 55(2):182-196.
Veerasakul S, Thanoi S, Reynolds GP and Nudmamud-Thanoi S. Effect of Methamphetamine Exposure on Expression of Calcium Binding Proteins in Rat Frontal Cortex and Hippocampus. Neurotoxicity Research 2016; 30:427–433.
Segal DS, Kuczenski R, O'Neil ML, Melega WP and Cho AK. Escalating Dose Methamphetamine Pretreatment Alters the Behavioral and Neurochemical Profiles Associated with Exposure to a High-Dose Methamphetamine Binge. Neuropsychopharmacology 2003; 28(10):1730–1740.
Parrilla I, Perez-Patiño C, Li J, Barranco I, Padilla L, Rodriguez-Martinez H, et al. Boar semen proteomics and sperm preservation. Theriogenology 2019; 137: 23-29.
Hongxia Z, Yin L, Bin L, Shengmin Y, Xuejiang G and Jiayin D. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. Journal of proteome research 2014; 13(7): 3370-3385.
Gill SS, Mueller RW, McGuire PF. and Pulido OM. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicologic Pathology 2000; 28: 277–284.
Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, Dell’Albani P, et al. Expression of metabotropic glutamate receptors in the rat and human testis. Journal of Endocrinology 2001; 170: 71–78.
Thanoi S, Janphet S and Nudmamud-Thanoi S. Changes of dopamine D2, alpha1 adrenergic receptor expressions and developmental stages of seminiferous tubule in rat testis after methamphetamine administration: A preliminary study. Songklanakarin Journal Science Technology 2020; 42 (4):928-934.
Huo S, Zhong X, Wu X and Li Y. Effects of norepinephrine and acetylcholine on the development of cultured Leydig cells in mice. Journal Biomedicine Biotechnology 2012; 503093:2.
Mhaouty-Kodja S, Lozach A, Habert R, Tanneux M, Guigon C, Brailly-Tabard S, et al. Fertility and spermatogenesis are altered in {alpha}1 b adrenergic receptor knockout male mice. Journal of Endocrinology 2007; 195:281–292.
Way AL and Killian GJ. Capacitation and induction of the acrosome reaction in bull spermatozoa with norepinephrine. Journal of Andrology 2002; 23:352–357.
Cornett LE and Meizel S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proceedings of the National Academy of Sciences 1978; 75:4954–4958.
Bavister BD, Chen AF and Fu PC. Catecholamine requirement for hamster sperm motility in vitro. Journal of reproduction and fertility 1979; 56:507–513.
Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacological Research 2004; 50:545–549.
Bruno PM, Pedro FO, and Marco GA. Molecular Mechanisms Controlled by mTOR in Male Reproductive System. International Journal of Molecular Sciences 2019; 20(7): 1633.
Hao X, Lianju S, Xuemei C, Yubin D, Junlin H, et al. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reproductive BioMedicine Online 2016; 32:207–217.
Yamada M, Monden T, Konaka S and Mori M. Assignment of human thyrotropin-releasing hormone (TRH) receptor gene to chromosome 8. Somatic Cell and Molecular Genetics 1993; 19 (6):577–80.
Zhao Y, Hou GW, Zhu HP, Zhao J, Wang RA, et al. Expression of thyrotropin-releasing hormone receptors in rat testis and their role in isolated Leydig cells. Cell and Tissue Research 2008; 334: 283–294.
Fukusumi S, Ogi K, Onda H and Hinuma S. Distribution of thyrotropin-releasing hormone receptor mRNA in rat peripheral tissues. Regulatory Peptides 1995; 57(2):115-121.
Khan UW and Rai U. Differential effects of histamine on Leydig cell and testicular macrophage activities in wall lizards: precise role of H1/H2 receptor subtypes. Journal of Endocrinology 2007; 194(2):441-448.
Mondillo C. Histamine in the testicle: new functions through classical H1 and H2 receptors. Revista Internacional de Andrología 2011; 9(2):54-61.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







