Pomegranate peel water extract induces apoptosis in breast cancer cells
Keywords:
Pomegranate, Antiproliferation, DNA fragmentation, Apoptosis, Breast cancerAbstract
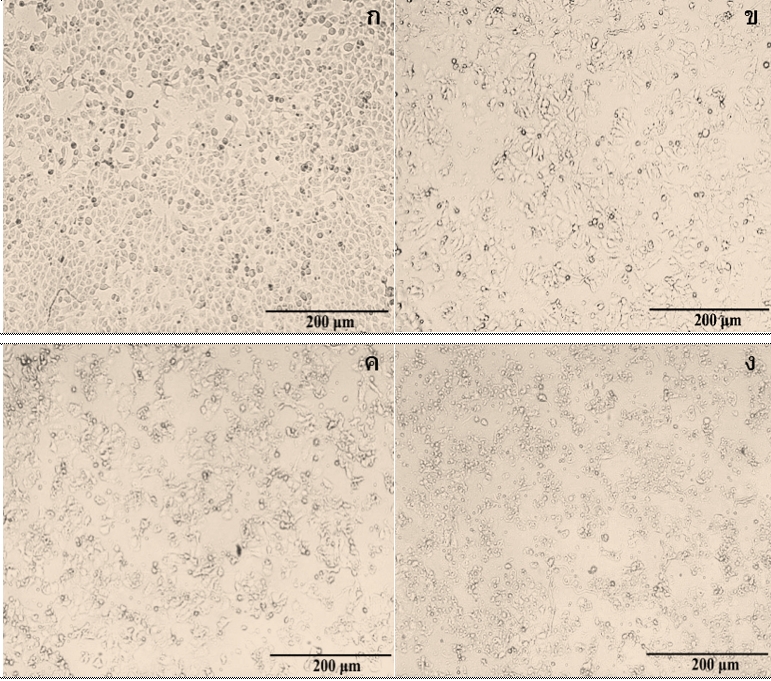
Breast cancer is more common in females, which this illness needs special care and treatment that makes them expensive. Medicinal plants, especially pomegranates, offer another choice to deal with the impacts of critical illnesses with lower costs of the treatment, which the utilization have been documented in traditional medicine. In this research, pomegranate peel water extract (PWE) was explored for many perspectives associating with the caspase-3-deficient MCF-7 breast cancer cells, particularly on the antiproliferative effects, DNA fragmentation, and apoptotic protein-induced cell death such as bcl-2, procaspase-9, procaspase-7, and poly (ADP-ribose) polymerase (PARP). The result found that PWE could inhibit the MCF-7 cell proliferation in a dose- and time-dependent manner, which was seen from an induction of antiproliferation on MCF-7 cells via apoptosis. This apoptosis correlated with the reductions of bcl-2, procaspase-9, and procaspase-7, and the activation of cleaved-PARP molecule activity. Moreover, these protein expressions associated with morphological change and DNA fragmentation in the MCF-7 cells. In conclusion, the PWE showed an inhibitory effect on proliferation of MCF-7 breast cancer cells by inducing DNA fragmentation and apoptosis through the expression of influencing apoptotic proteins.
References
Merril RP, Weed DL. Measuring the public health burden of cancer in the United States through lifetime and age-condition rich estimates. Ann Epidemiol. 2001;11:547-533.
Yip CH. Breast cancer in Asia. Methods Mol Biol. 2009;471:51-64.
Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780.
Haskell CM. Principles of cancer chemotherapy. In: Cancer Treatment. 2nd ed. Haskell CM., (ed). Philadelphia: WB. Saunders. p. 21-42.
Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999;158:207-210.
Woraratphoka J, Intarapichet KO, Indrapichate K. Antioxidant activity and cytotoxicity of six selected, regional, Thai vegetables. American-Eurasian J Toxic Sci. 2012;4(2):108-117.
Manasathien J. Effects of mangosteen hull extracts on bioefficacy and antiproliferation of human breast and prostate carcinoma cell lines. Suranaree J Sci Technol. 2017;24(4):475-488.
Morton JF. Fruits of warm climates. 1987. Miami: FL. p. 352-355.
Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177-206.
Bianchini F, Corbetta F. Health plants of the world. 1979. New York: Newsweek.
Duke AJ, Ayensu SE. Medicinal plants of China. 1985. Reference Publications: MI.
de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem. 2000;48:5331-5337.
van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J. Rapid dereplication of estrogen compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry. 2004;65:233-241.
Noda Y, Kaneyuka T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyaniding, and pelargonidin. J Agric Food Chem. 2002;50:166-171.
Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581-4589.
Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Noro T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol Pharm Bull. 1993;16:787-790.
Tanaka T, Nonaka GI, Nishioka I. Tannins and related compounds. C. Reaction of dehydrohexahydroxydiphenic acid esters with bases, and its application to the structure determination of pomegranate tannins, granatins a and b. Chem Pharm Bull. 1990;38:9424-9428.
Guo CJ, Yang JJ, Wei JY, Li YF, Xu J, Jiang YG. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;31:1719-1726.
Manasathien J, Kupittayanant S, Indrapichate K. Protective efficacy of pomegranate (Punica granatum Linn., Punicaceae) peel ethanolic extract on UVB-irradiated rat skin. Am-Eurasian J Toxicol Sci. 2011;3(4):250-258.
Lansky EP, Harrison G, Froom P, Jiang WG. Pomegranate (Punica granatum) pure chemicals show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Martigel. Invest New Drugs. 2005;23:121-122.
Lansky EP, Jiang W, Mo H, Bravo L, Froom P, Yu W. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Invest New Drugs. 2005;23:11-20.
Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7:274-283.
Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163-173.
Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980-985.
Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360-367.
Toi M, Banado H, Ramachandran C, Melnick SJ, Imai A, Fife RS, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6:121-128.
Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71:203-217.
Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611-625.
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219.
Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543-8567.
Korsmeyer SJ. Bcl-2: an antidote to programmed cell death. Cancer Res. 1992;53:4251-4256.
Manasathien J, Indrapichate K. Apoptosis of MCF-7 cancer cell induced by pomegranate (Punica granatum L.) peel extract. Suranaree J Sci Technol. 2016;21(1):63-74.
Manasathien J, Indrapichate K, Intarapichate K. Antioxidant activity and bioefficacy of pomegranate Punica granatum Linn. peel and seed extracts. Global J Pharm. 2012;6(2):131-141.
Hansen M, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203-210.
Goegan P, Johnson G, Vincent R. Effects of serum protein and colloid on the alamarBlue assay in cell cultures. Toxicol In Vitro. 1995;9:257-266.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254.
Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334-340.
Ackland ML, van de Waarsenburg S, Jones R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo. 2005;19:69-76.
Aslam MN, Lansky EP, Varani J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J Ethnopharmacol. 2006;103:311-318.
Coates EM, Popa G, Gill CIR, McCann MJ, McDougall GJ, Stewart D, Rowland I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J Carcinog. 2007;6:4.
Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis caspase-3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274(33):22932-22940.
Wolf BB, Schuler M, Escheverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274:30651-30656.
Walsh JG, Cullen SP, Sheridan C, Luthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. P Natl Acad Sci USA. 2008;105:12815-12819.
Sakakura C, Sweeney EA, Shirahama T, Igarashi Y, Hakomori S, Tsujimoto H, et al. Overexpression of Bax sensitizes breast cancer MCF-7 cells to cisplatin and etoposide. Jpn J Surg. 1997;27:676-679.
Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357-9360.
Kagawa S, Gu J, Honda T, McDonnell TL, Swisher SG, Roth JA, et al. Deficiency of caspase-3 in MCF-7 cells blocks bax-mediated nuclear fragmentation but not cell death. Clin Cancer Res. 2001;7:1474-1480.
Hsuuw YD, Chan WH. Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann NY Acad Sci. 2007;1095:428-440.
Siemankowski LM, Morreale J, Briehl MM. Antioxidant defenses in TNF-treated MCF-7 cells: selective increase in MnSOD. Free Radical Bio Med. 1999;26:919-924.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312.
Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. P Natl Acad Sci USA. 2005;102:14813-14818.
Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950-2966.
Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296(5573):1635-1636.
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;367:37-43.
Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase mediated activation of PAK2. Science. 1997;276:1571-1574.
Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056-26061.
Lakhani SA, Masud A, Kuida K, Porter GA, Booth CJ, Mehal WZ, et al. Caspase 3 and 7: Key mediators of mitochondrial events of apoptosis. Science. 2006;311:847-851.
Leonard JR, Klocke BJ, D’Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropath Exp Neur. 2002;61:673-677.
Houde C, Banks KG, Coulombe N, Rasper D, Grimm E, Roy S, et al. Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of caspase-3-null mice. J Neurosci. 2004;24:9977-9984.
Mc Gee MM, Hyland E, Campiani G, Ramunno A, Nacci V, Zisterer DM. Caspase-3 is not essential for DNA fragmentation in MCF-7 cells during apoptosis induced by the pyrrolo-1,5-benzoxazepine, PBOX-6. FEBS Lett. 2002;515:66-70.
Lee SH, Jaganath IB, Wang SM, Sekaran SD. Antimetastatic effects of Phyllanthus on human lung (A549) and breast (MCF-7) cancer cell lines. Plos ONE. 2011;6(6):e20994.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







