Insoluble phosphate solubilisation and acid phosphatase activity of bacteria isolated from organic paddy soils
Keywords:
Organic paddy soils, Phosphate-solubilising bacteria, Tricalcium phosphate solubilisation, Organic phosphorus mineralisation, Acid phosphatase activityAbstract
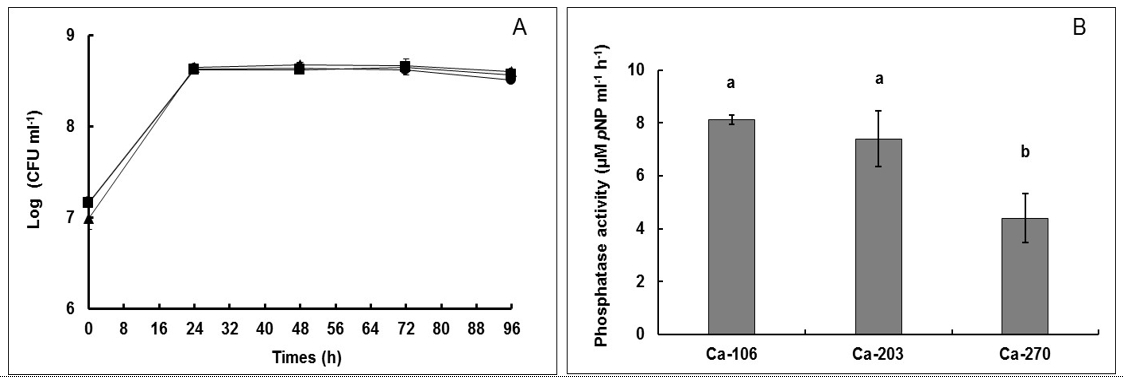
An increase in phosphorus (P) availability through the addition of microbial inoculant, such as phosphate-solubilising bacteria (PSB), in organic agricultural soils, could be a good option for increasing soil fertility. We evaluated phosphate-solubilising (PS) ability and phosphate-mineralising (PM) ability of PSB isolated from organic paddy soils. Total soil bacteria were isolated on LB agar and tested on NBRIP agar for tricalcium phosphate (TCP) solubilisation. Three PSB strains were selected, based on their colonial morphologies on LB agar, for the measurement of PS ability in NBRIP broth, and PM ability in the presence of acid phosphatase substrate, p-nitrophenyl phosphate in culture broth. PSB were identified by morphology and a molecular technique using 16S rRNA gene. The results indicated that the selected PSB were able to solubilise TCP and presented acid phosphatase activity. The TCP solubilisation was performed via acid production in NBRIP broth. The strain Ca-106, which was closely related to Bacillus sp. based on the identity of 16S rRNA gene sequences, presented the TCP solubilisation of 181.57 mg P l-1 which was higher than that in the previous report. The other strains (Ca-203 and Ca-270) solubilised TCP with the values of 195.48 mg P l-1 and 180.46 mg P l-1 respectively. They were closely related to Enterobacter sp., based on the identity of 16S rRNA gene sequences. The activity of extracellular acid phosphatase presented the values of 8.12 µM pNP ml-1 h-1, 7.41 µM pNP ml-1 h-1 and 4.40 µM pNP ml-1 h-1 for the strains Ca-106, Ca-203 and Ca-270, respectively. The high activity of acid phosphatase was observed for the selected PSB when compared to the previous reports that used the same technique. Taken together, the results indicated that all selected PSB had both PS ability via the acidification process and PM ability via the production of extracellular acid phosphatase. They can be potentially applied as microbial inoculants for increasing, especially, organic P mineralisation in organic agricultural soils. Further, the PSB strains merit a further study with a target plant, in pot experiments, in order to verify their ability to promote plant growth.
References
Anderson G. Assessing organic phosphorus in soils. In: Khasawneh FE, Sample EC, Kamprath EJ, editors. The role of phosphorus in agriculture. Wisconsin: American Society of Agronomy. 1980. p. 411–431.
Hinsinger P, Herrmann L, Lesueur D, Robin A, Trap J, Waithaisong K, Plassard C. Impact of roots, microorganisms, and microfauna on the fate of soil phosphorus in the rhizosphere. In: Lambers H, William CP, editors. Annual plant reviews, volume 48, phosphorus metabolism in plants. New York: Wiley-Blackwell. 2015. p. 377–398.
Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil. 2001; 237(2): 173–195.
Rodríguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999; 17(4-5): 319–339.
Jones DL, Oburger E. Solubilization of phosphorus by soil microorganisms. In: Bünemann E, Oberson A, Frossard E, editors. Phosphorus in action. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. 169-198.
Billah M, Khan M, Bano A, Hassan TU, Munir A, Gurmani AR. Phosphorus and phosphate solubilizing bacteria: keys for sustainable agriculture. Geomicrobiol. J. 2019;36(10):904–916.
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010;60(4): 579–598.
Plassard C, Robin A, Le Cadre E, Marsden C, Trap J, Herrmann L, Waithaisong K, Lesueur D, Blanchart E, Chapuis-Lardy L, Hinsinger P. Améliorer la biodisponibilité du phosphore : comment valoriser les compétences des plantes et les mécanismes biologiques du sol ?. Innov. Agron. 2015; 43: 115–138.
Rodríguez H, Fraga R, Gonzalez T, Bashan Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil. 2006; 287: 15–21.
Glick B. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012; 2012: 963401.
Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8: 971.
Gomiero T, Pimentel D, Paoletti MG. Environmental impact of different agricultural management practices: conventional vs. organic agriculture Tiziano. CRC. Crit. Rev. Plant Sci. 2011;30(1-2):95–124.
Willer H, Kicher L. The world of organic agriculture : statistics and emerging trends, FiBL-IFOAM Report, IFOAM, 1st ed. Bonn and FiBL: Frick; 2011. 34 p.
Mäder P, Fließbach A, Dubois D, Gunst L, Fried P, Niggli U. Soil fertility and biodiversity in organic farming. Science. 2002; 296(5573): 1694–1697.
De Ponti T, Rijk B, Van Ittersum MK. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012;108:1–9.
Seufert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. 2012;485(7397): 229–232.
Entz MH, Guilford R, Gulden R. Crop yield and soil nutrient status on 14 organic farms in the eastern portion of the northern Great Plains. Can. J. Plant Sci. 2001;81(2): 351–354.
Gosling P, Shepherd M. Long-term changes in soil fertility in organic arable farming systems in England, with particular reference to phosphorus and potassium. Agric. Ecosyst. Environ. 2005;105(1-2): 425–432.
Möller K, Oberson A, Bünemann EK, Cooper J, Friedel JK, Glæsner N, et. al. Improved phosphorus recycling in organic farming: navigating between constraints. In: Sparks DL, editors. Advances in agronomy, volume 147. London: Academic Press; 2018. p. 159–237.
Jorquera MA, Hernández MT, Rengel Z, Marschner P, De La Luz Mora M. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils. 2008;44(8):1025–1034.
Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170(1):265–270.
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008; 74(8):2461–2470.
Bell TG, Kramvis A. Fragment merger: An online tool to merge overlapping long sequence fragments. Viruses. 2013;5(3):824–833.
Johri JK, Surange S, Nautiyal CS. Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils. Curr. Microbiol. 1999;39(2):89–93.
American Society for Microbiology. Preparation of routine media and reagents used in antimicrobial susceptibility testing. Available from: https://www.asmscience.org [accessed 31 January 2018].
Ohno T, Zibilske LM. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991; 55(3): 892–895.
De Freitas JR, Banerjee MR, Germida JJ. Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol. Fertil. Soils. 1997;24(4):358–364.
Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1(4):301–307.
Li Y, Zhang J, Zhang J, Xu W, Mou Z. Characteristics of inorganic phosphate-solubilizing bacteria from the sediments of a eutrophic lake. Int. J. Environ. Res. Public Health. 2019; 16(12): 2141.
Vasanthi N, Saleena LM, Raj SA. Silica solubilization potential of certain bacterial species in the presence of different silicate minerals. Silicon. 2018; 10(2): 267–275.
Collavino MM, Sansberro PA, Mroginski LA, Aguilar OM. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils. 2010; 46(7): 727–738.
Mendoza-Arroyo GE, Chan-Bacab MJ, Aguila-Ramírez RN, Ortega-Morales BO, Solís REC, Chab-Ruiz AO, et al. Inorganic phosphate solubilization by a novel isolated bacterial strain Enterobacter sp. Itcb-09 and its application potential as biofertilizer. Agric. 2020; 10(9): 383.
Silva CF, Vitorino LC, Mendonça MAC, Araújo WL, Dourado MN, Albuquerque LC, et. al. Screening of plant growth-promoting endophytic bacteria from the roots of the medicinal plant Aloe vera. South African J. Bot. 2020; 134: 3–16.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







