Preliminary in vitro investigation of Litsea petiolata extracts for sustainable control of bacterial leaf blight in rice caused by Xanthomonas oryzae pv. oryzae
Keywords:
Litsea petiolata, Bacterial leaf blight disease, Antibacterial activity, Plant extract, Biological controlAbstract
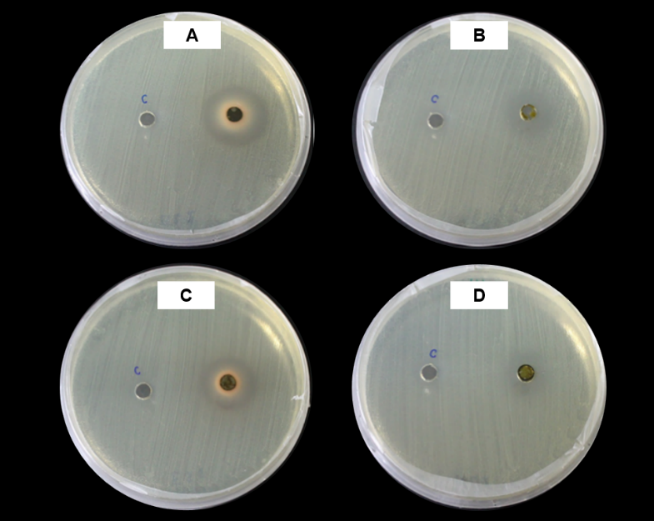
Litsea petiolata, locally called Tham Mung, is widely distributed across tropical and subtropical regions of Asia, and North and South America. This plant is notable for its ecological significance and cultural relevance. Renowned for its rich secondary metabolites, especially phenolic compounds, L. petiolata has been studied for its medicinal and antimicrobial properties. This study investigates the phenolic content and antibacterial activity of ethanol and hexane extracts from the leaves and stems of L. petiolata, specifically targeting Xanthomonas oryzae pv. oryzae (Xoo), the bacterium responsible for bacterial leaf blight in rice. High-performance liquid chromatography identified key phenolic compounds, including vanillic acid in the leaves and 4-hydroxybenzoic acid in the stems. The antibacterial efficacy of these extracts was evaluated using clear zone assays, minimum inhibitory concentration, and minimum bactericidal concentration testing. The results indicated that the ethanol extract from the leaves exhibited the most potent antibacterial activity, significantly inhibiting the growth of Xoo. These findings suggest that L. petiolata holds considerable potential as a source of natural antibacterial agents, offering an environmentally sustainable alternative to chemical treatments for managing bacterial diseases of rice.
References
Mahattanatawee K, Luanphaisarnnont T, Rouseff R. Comparison of aroma character impact volatiles of Thummong leaves (Litsea petiolata Hook. f.), Mangdana water beetle (Lethocerus indicus), and a commercial product as flavoring agents in Thai traditional cooking. J Agric Food Chem. 2018;66(10):2480-4.
Goh MP, Samsul RN, Mohaimin AW, Goh HP, Zaini NH, Kifli N, et al. The analgesic potential of Litsea species: A systematic review. Molecules. 2024;29(9):2079.
Wang L, Hu W, Deng J, Liu X, Zhou J, Li X. Antibacterial activity of Litsea cubeba essential oil and its mechanism against Botrytis cinerea. RSC Adv. 2019;9(50):28987-95.
Atiar RM, Anjum N, Khalid JRM, Saha S, Ibne DJ, Uddin M, et al. Pyrazoles containing organic extracts of Litsea glutinosa (Lour.) C. B. Rob enervate chemical-induced diarrhea in animal models evident in ligand-receptor interaction. Arab J Chem. 2023;16(8):104910.
Vishakha K, Das S, Das SK, Banerjee S, Ganguli A. Antibacterial, anti-biofilm, and anti-virulence potential of tea tree oil against leaf blight pathogen Xanthomonas oryzae pv. oryzae instigates disease suppression. Braz J Microbiol. 2022;53(1):19-32.
Li S, Jiang S, Jia W, Guo T, Wang F, Li J, et al. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2024;432:137231.
Ben FL, Romeo FV, Foti P, Russo N, Randazzo CL, Caggia C, et al. Multi-functional potential of lactic acid bacteria strains and antimicrobial effects in minimally processed pomegranate (Punica granatum L. cv Jolly Red) arils. Microorganisms. 2022;10(10):1876.
Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321-4.
Tourabi M, Metouekel A, ghouizi AEL, Jeddi M, Nouioura G, Laaroussi H, et al. Efficacy of various extracting solvents on phytochemical composition, and biological properties of Mentha longifolia L. leaf extracts. Scientific Reports. 2023;13(1):18028.
Abbas A, Naqvi SAR, Rasool MH, Noureen A, Mubarik MS, Tareen RB. Phytochemical analysis, antioxidant and antimicrobial screening of Seriphidium oliverianum plant extracts. Dose-Response. 2021;19(1):15593258211004739.
Sangsila A, Chumroenphat T, Jorjong S. Effects of drying methods on active odorants, phytochemicals and antioxidant properties of Litsea petiolata Hook. f. leaves locally used as a substitute to male giant water bugs in pungent chili pastes. Int J Agric Technol. 2021;17(4):1577-90.
Paiva L, Lima E, Motta M, Marcone M, Baptista J. Influence of seasonal and yearly variation on phenolic profiles, caffeine, and antioxidant activities of green tea (Camellia sinensis (L.) Kuntze) from Azores. App Sci. 2021;11(16):7439.
Faleva AV, Ulyanovskii NV, Onuchina AA, Kosyakov DS. Polyphenolic antioxidants in bilberry stems and leaves: A non-targeted analysis by two-dimensional NMR spectroscopy and liquid chromatography–hgh-resolution mass spectrometry. Antioxidants. 2024;13(11):1409.
Ha TTP, Tran TBN, Tram TNN, Kha VH. Total phenolic, total flavonoid contents and antioxidant potential of common bean (Phaseolus vulgaris L.) in Vietnam. AIMS Agric Food. 2020;5(4):635-48.
Nakurte I, Berga M, Mežaka I. Phytochemical diversity comparison in leaves and roots of wild and micropropagated Latvian sea holly (Eryngium maritimum L.). Molecules. 2023;28(9):3924.
Isah T. Stress and defense responses in plant secondary metabolites production. Biol Res. 2019;52(39).
Kuljarusnont S, Iwakami S, Iwashina T, Tungmunnithum D. Flavonoids and other phenolic compounds for physiological roles, plant species delimitation, and medical benefits: A promising view. Molecules. 2024;29(22):5351.
Mutuku JM, Cui S, Hori C, Takeda Y, Tobimatsu Y, Nakabayashi R, et al. The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiol. 2019;179(4):1796-809.
Wulandari I, Kusuma IW, Kuspradini H. Antioxidant and antibacterial activity of Litsea garciae. IOP Conf Ser Earth Environ Sci. 2018;144(1):012024.
Hai TN, Trang HD, Ha TNT. Herbal extracts in combination with nanosilver inhibit blight disease caused by Xanthomonas oryzae pv. oryzae in rice. VJAS. 2018;1(4): 270-80.
Kurç AM, Orak HH, Gülen D, Caliskan H, Argon M, Sabudak T. Antimicrobial and antioxidant efficacy of the lipophilic extract of Cirsium vulgare. Molecules. 2023;28(20):7177.
Hu W, Li C, Dai J, Cui H, Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind Crops Prod. 2019;130:34-41.
Bai X, Chen T, Liu X, Liu Z, Ma R, Su R, et al. Antibacterial activity and possible mechanism of Litsea cubeba essential oil against Shigella sonnei and its application in lettuce. Foodborne Pathog Dis. 2023;20(4):138-48.
López-Romero JC, González-Ríos H, Peña-Ramos A, Velazquez C, Navarro M, Robles-Zepeda R, et al. Seasonal effect on the biological activities of Litsea glaucescens Kunth extracts. Evid Based Complement Alternat Med. 2018;2018(1):2738489.
Kuspradini H, Wulandari I, Putri AS, Tiya SY, Kusuma IW. Phytochemical, antioxidant and antimicrobial properties of Litsea angulata extracts. F1000Res. 2018;22(7):1839.
Maisch NA, Bereswill S, Heimesaat MM. Antibacterial effects of vanilla ingredients provide novel treatment options for infections with multidrug-resistant bacteria – A recent literature review. Eur J Microbiol Immunol. 2022;12(3):53-62.
Yujia L, Shi C, Zhang G, Zhan H, Liu B, Li C, et al. Antimicrobial mechanism of 4-hydroxyphenylacetic acid on Listeria monocytogenes membrane and virulence. Biochem Biophys Res Commun. 2021;572:145-50.
Ecevit K, Barros AA, Silva JM, Reis RL. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022;2(4):460-98.
Kim Y, Zhao H, Avena-Bustillos RJ, Wang SC, Nitin N. Synergistic antimicrobial activities of aqueous extract derived from olive byproduct and their modes of action. Chem Biol Technol Agric. 2024;11(1):122.
Ramirez DA, Altamirano JC, Camargo AB. Multi-phytochemical determination of polar and non-polar garlic bioactive compounds in different food and nutraceutical preparations. Food Chem. 2021;337:127648.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 University of Phayao

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







