Effects of medium components on in vitro multiplication efficiency of Siam tulip
Keywords:
Cytokinins, Shoot multiplication, Concentration of mediumAbstract
Siam tulip (Curcuma alismatifolia) is a significant economic ornamental plant in Thailand. A commercial production of Siam tulip should mainly use disease-free rhizome to avoid disease accumulation, which could affect the export of Siam tulip’s rhizomes. Therefore, the enhancing efficiency of tissue culture of Siam tulip for disease-free and quality-consistent in plant production is another approach to improve the quality of Siam tulip production for export capacity. This study aimed to evaluate the effects of cytokinins and nutrient concentrations in MS medium, by using shoots harvested from cv. Red Shadow and cv. Khao Yai as explant for the experiments. The results showed that the 4 mg/L of N6-benzylaminopurine (BAP) resulted in the highest shoot multiplication of cv. Khao Yai and 5 mg/L of BAP gave the highest shoot multiplication of cv. Red Shadow. While Kinetin resulted in the increasing of length of explants. Moreover, the reduction of nutrient concentration in MS medium to 0.5 times and the increase of sucrose concentration to 2 times or 6% provided the highest shoot multiplication. While reducing the nutrient concentration in MS medium to 0.25 times decreased the growth of both cv. Red Shadow and cv. Khao Yai.
References
Lekawatana S, Suwannamek B. Ornamental plants in Thailand. Proceeding of the I International Symposium on Tropical and Subtropical Ornamentals, 2016 Mar 7-9; Krabi, Thailand. Acta Hortic. 1167. 2016;1-16.
Ruamrungsri S. The physiology of Curcuma alismatifolia Gagnep. as a basis for the improvement of ornamental production. Eur. J. Hortic. Sci. 2015;80(6):316-321.
Boontiang K, Siritrakulsak T, Nontaswatsri C. A strategic approach to achieve healthy plant growth and decontaminated rhizome of Curcuma alismatifolia Gagenep. cultivation in modified substrate on raised-bed planting. J. Appl. Hortic. 2024;26(02):202-205.
Promsai S, Tragoolpua Y, Thongwai N. Biological control of bacterial wilt in Pathumma; Curcuma alismatifolia. Glob. J. Environ. Sci. Manag. 2023;9:127-144.
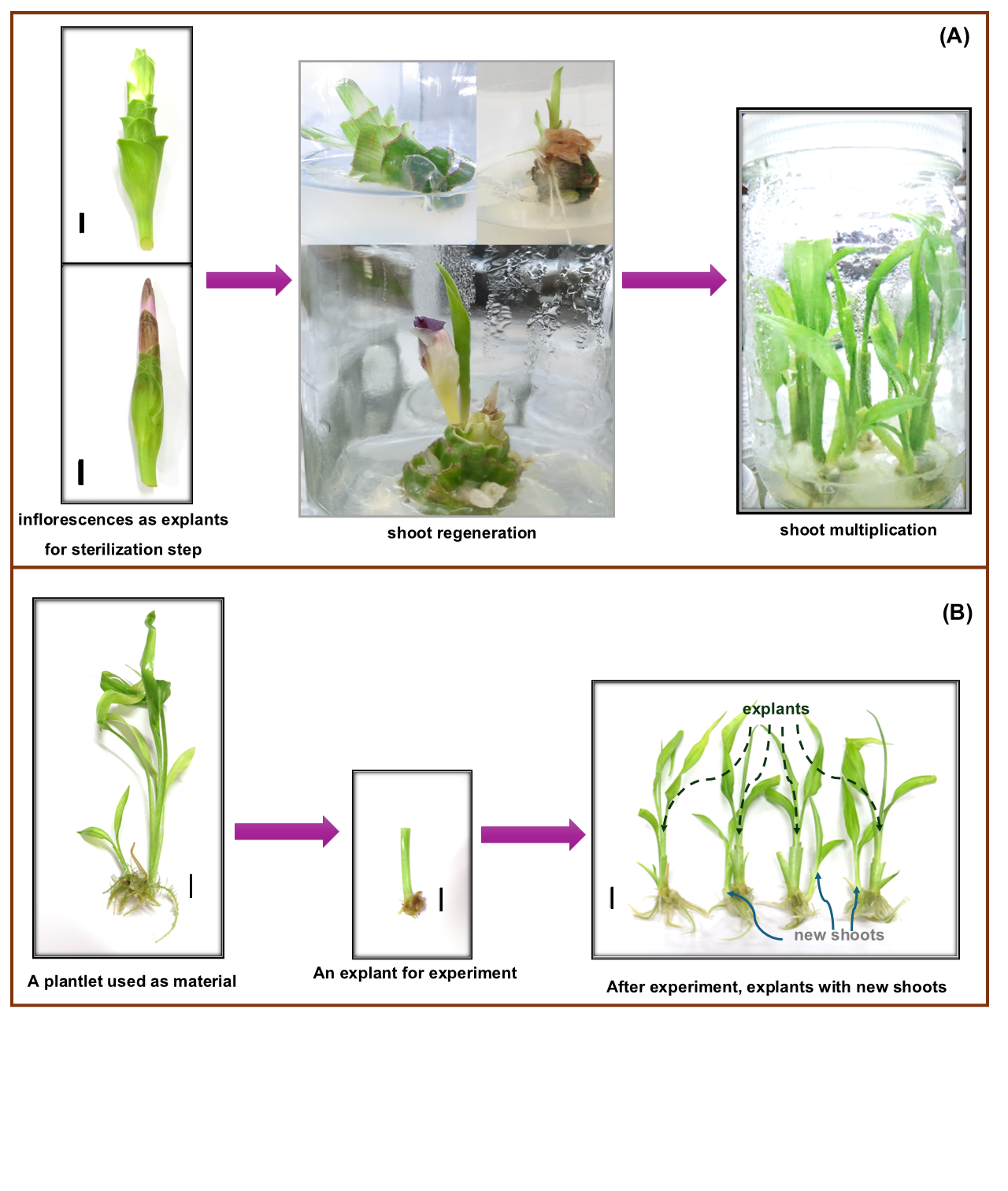
Toppoonyanont N, Chongsang S, Chujan S, Somsueb S, Nuamjaroen P. Micropropagation Scheme of Curcuma alismatifolia Gagnep. In IX International Symposium on Flower Bulbs. Acta Hortic. 673. 2004;705-712.
Zhang S, Liu N, Sheng A, Ma G, Wu G. Direct and callus-mediated regeneration of Curcuma soloensis Valeton (Zingiberaceae) and ex vitro performance of regenerated plants. Sci. Hortic. 2011;130(4):899-905.
Van Tan P. Micropropagation of Curcuma sp., a threatened medicinal plant. Adv. Biosci. Biotechnol. 2016;7(10):418.
Yoosumran V, Saetiew K, Ruamrungsri S, Akarapisarn A, Teerarak M. Micropropagation of young inflorescence Curcuma hybrid in vitro. Int. J. Agric. Technol. 2022;18(3):1355-1366.
Hung C D, Trueman S J. Cytokinin concentrations for optimal micropropagation of Corymbia torelliana× C. citriodora. Aust. For. 2012;75(4):233-237.
Sarkar J, Banerjee N. Influence of different cytokinins on micropropagation of an important medicinal plant, Solanum erianthum D. Don, and assessment of the genetic fidelity of the regenerants. In Vitro Cell Dev. Biol-Plant. 2020;56:480-490.
Poothong S, Khen T, Chumphukam O. In vitro mineral nutrition for improving growth and multiplication of stevia. Agr. Nat. Resour. 2018;52(5):477-483.
Khajehyar R, Tripepi R, Price W J, Love S. Optimization of Selected Minerals and a Cytokinin for In Vitro Propagation of Little-Leaf Mockorange (Philadelphus microphyllus A. Gray) Using Response Surface Methodology (RSM). Plants. 2024;13(23):3446.
Poothong S, Reed B M. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci. Hortic. 2014;165:132-141.
Ayoola-Oresanya I O, Sonibare M A, Gueye B, Abberton M T, Morlock G E. Elicitation of antioxidant metabolites in Musa species in vitro shoot culture using sucrose, temperature and jasmonic acid. Plant Cell Tissue Organ Cult. 2021;146:225-236.
Jo E A, Tewari R K, Hahn E J, Paek K Y. In vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell Tissue Organ Cult. 2009;96:307-315.
Al-Khalifah N S, Hadi S, Khan F. Influence of Sucrose Concentration on in vitro Growth of Five Rose (Rosa hybrida L.) Cultivars. Plant Tissue Cult. 2005;15(1):43-49.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497.
George E F, Hall M A, De Klerk G J, editors. Plant propagation by tissue culture, 3rd ed. Basingstoke: Springer Dordrecht; 2008.502 p.
Saensouk S, Sonthongphithak P, Chumroenphat T, Muangsan N, Souladeth P, Saensouk P. In Vitro Multiplication, Antioxidant Activity, and Phytochemical Profiling of Wild and In Vitro-Cultured Plants of Kaempferia larsenii Sirirugsa—A Rare Plant Species in Thailand. Hortic. 2025;11(3):281.
Noor Camellia N A, Nora’ini A, Izlamira R, Mirfat A H S, Ahmad Arif I, Yaseer S. The Impact of 6-Benzylaminopurine (BAP) on Plant Growth, Micro-Rhizome Induction and Phytochemical Content of Black Ginger (Kaempferia Parviflora) Using Two In-Vitro Culture System. J. adv. biol. 2025;28(2):526-36.
Bhattacharya M, Sen A. Rapid in vitro multiplication of disease-free Zingiber officinale Rosc. Indian J. Plant Physiol. 2006;11(4):379-384.
Ugochukwu S C, Bob S E, Ozioma O, Odii E B, Ijeoma I C, Olanike O. Shoot proliferation of in vitro turmeric (Curcuma longa L.) affected by different concentrations of benzylaminopurine (BAP). No. of surviving plantlets X 100No. replicates in a treatment. World J. Agric. Sci. 2013;9(3):227-230.
Abu-Romman S M, Al-Hadid K A, Arabiyyat A R. Kinetin is the most effective cytokinin on shoot multiplication from cucumber. J. Agric. Sci. 2015;7(10):159.
Zapata E V, Morales G S, Lauzardo A N H, Bonfil B M, Tapia G T, Sánchez A D J, et al. In vitro regeneration and acclimatization of plants of turmeric (Curcuma longa L.) in a hydroponic system. Biotecnol. apl. 2003;20(1):25-31.
Taghavi T, Rahemi A, Rafie R, Kering M K. Optimizing turmeric tissue culture, testing different media and a plant growth regulator matrix. Hort Tech. 2021;31(6):692-704.
Haida Z, Sinniah U R, Nakasha J J, Hakiman M. Shoot induction, multiplication, rooting and acclimatization of black turmeric (Curcuma caesia Roxb.): an important and endangered curcuma species. Hortic. 2022;8(8):740.
Zuraida A R. Improved in vitro propagation of Curcuma caesia, a valuable medicinal plant. J. Trop. Agric. and Fd. Sc. 2013;41(2):273 – 281.
Poothong S. Optimization of minerals and plant growth regulators for micropropagation of strawberry ‘Pharachatan 80’. Health Sci. Tech. Rev. 2020;13(2):5-17.
Gezahegn G, Feyisa T, Rezene Y. Induction of micro-rhizomes for in vitro ginger (Zingiber officinale Rosco) disease-free planting materials regeneration. Biotechnol. Rep. 2024;41:e00820.
Al-Khateeb A. Influence of different carbon sources and concentrations on in vitro root formation of date palm, Phoenix dactylifera L. cv Khanezi. Zagazig. J Agricultural Res. 2002;28(3):597–608.
Gürel S, Gülsen Y. The effects of different sucrose, agar and pH levels on in vitro shoot production of almond (Amygdalus communis L.). Turkish J Bot. 1998;22:363–373.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 University of Phayao

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







