Analysis of Legal Factors Related to Drug Security in Thailand: Scoping Review
Keywords:
Access to medicine, Drug security, Drug supply chain, Emergency, Legal frameworkAbstract
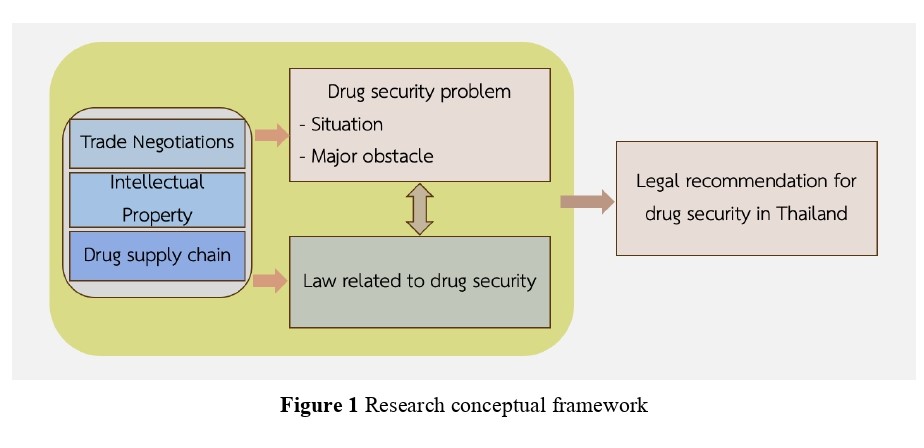
The United Nations Sustainable Development Goals (SDGs) related to health and well-being are closely connected to drug security, which in this study refers to “available when in need” for both normal and emergencies. This study was secondary research, employing a scoping review methodology to gather data on the legal factors affecting drug security issues in Thailand from January 1, 2000 to August 31, 2024. The data was gathered from academic databases and evaluated using the PRISMA-Scr Flow Diagram, alongside an exploration of legal awareness related to drug security through the Newscenter X news database. This study identified that the main issues affecting drug security in Thailand include reliance on imported pharmaceutical raw materials, inefficient management of the drug supply chain, the complexity of laws related to patents, trade competition, and international trade, which obstruct the production and access to generic drugs. Additionally, the system for procuring and supplying medicines during emergencies has proven inadequate in meeting demand promptly. Existing laws and measures require improvement to ensure drug security. The key recommendations from this study include the development of a new legal framework to support domestic drug production, procurement systems efficiency for greater flexibility and speed, and implementing compulsory licenses under patent laws when necessary to enhance access to affordable medicines for the people.
References
Akaleephan, C., Kiewnin, K., Sosom, J., & Hongtumrong, M. (2020)(a). An analysis on access to essential medicines in Thailand: the issues of policy incoherence. Health Systems Research Institute. https://kb.hsri.or.th/dspace/handle/11228/5269
Akaleephan, C., Leelahavarong, P., Yadee, J., Kumdee, C., Onjon, O., & Kiewnin, K. (2020)(b). A study on the supply management for inaccessible essential medicines under the Universal Health Coverage: system improvement. Health Systems Research Institute. https://kb.hsri. or.th/dspace/handle/11228/5198
Bangkokbiznews. (2013, 23 July ). Suggest the Government Pharmaceutical Organization stop competing with the private pharmaceutical industrial TPMA points out that it will affect the development of domestic manufacturers. Bangkokbiznews, 13
Bangkokbiznews. (2019, 25 October). ‘Anuthin’ commends policy to the Government Pharmaceutical Organization for drug security. Bangkokbiznews. https://www.bangkokbiznews.com/news/852123
Berkna, T., Srisorn, W., & Chayanon, S. (2021). National security on drugs and medical supplies in terms of production, procurement, research and medical supplies in critical situation of government pharmaceutical organization. Journal of MCU Social Science Review, 11(4), R55 - 68. https://so03.tci-thaijo.org/index.php/jssr/article/view/254703
CASP-UK. (2024). Critical Appraisal Skills Programme. CASP UK - OAP Ltd. https://casp-uk.net/casp-tools-checklists/
Cawthorne, P., Ford, N., Wilson, D., Kijtiwatchakul, K., Purahong, V., Tianudom, N., & Nacapew, S. (2007). Access to drugs: the case of Abbott in Thailand. The Lancet Infectious Diseases, 7(6), 373 - 374. https://doi.org/10.1016/s1473-3099(07)70118-4
Chungsivapornpong, W., Kittiratchakool, N., Kumluang, S., Onjon, O., & Tantivess, S. (2020). Drug procurement in public hospitals under the Public Procurement and Supplies Administration Act B.E. 2560 (2017). Journal of Health Systems Research, 14(3), 311 - 326. http://kb.hsri. or.th/dspace/handle/11228/5249
Committee of Thai Drug System Report. (2020). Thai drug system 2020. Health Systems Research Institute. https://kb.hsri.or.th/dspace/handle/11228/5234
D'Angelo, A. B., Grov, C., Johnson, J., & Freudenberg, N. (2021). Breaking bad patents: learning from HIV/AIDS to make COVID-19 treatments accessible. Glob Public Health, 16(10), 1523-1536. https://doi.org/10.1080/17441692.2021.1924223
Department of Disease Control. (2022, 29 September). Guideline for medical supplies management and resources in public health emergency. Department of Disease Control. https://ddc.moph. go.th/uploads/publish/1316120220929031951.pdf
Effingham, A. M. (2016). TRIPs agreement article 31(b): the need for revision. Seton Hall law review, 46(3), 883- 909. https://scholarship.shu.edu/cgi/viewcontent.cgi?article=1567& context=shlr
Ford, N., Wilson, D., Cawthorne, P., Kumphitak, A., Kasi‐Sedapan, S., Kaetkaew, S., Teemanka, S., Donmon, B., & Preuanbuapan, C. (2009). Challenge and co‐operation: civil society activism for access to HIV treatment in Thailand. Tropical Medicine & International Health, 14(3), 258-266. https://doi.org/10.1111/j.1365-3156.2009.02218.x
Koçkaya, G., Atalay, S., Oğuzhan, G., Kurnaz, M., Ökçün, S., Sar Gedik, Ç., Şaylan, M., & Şencan, N. (2021). Analysis of patient access to orphan drugs in Turkey. Orphanet J Rare Dis, 16(1), 68. https://doi.org/10.1186/s13023-021-01718-3
Kuek, V., Phillips, K., & Kohler, J. C. (2011). Access to medicines and domestic compulsory licensing: learning from Canada and Thailand. Global Public Health, 6(2), 111 - 124. https://doi.org/10.1080/17441690903575255
Le, V. A., & Hyland, M. (2020). Compulsory licensing for patented medicines: a comparative legal analysis of India, Brazil and Thailand. Manchester Journal of International Economic Law, 17(3), 315 - 337. https://heinonline.org/HOL/P?h=hein.journals/mjiel17&i=331
Leelaprathuang, N. (2021). The impact of CPTTP on accessing medicine in Thailand. Journal of Humanities and Social Sciences Suratthani Rajabhat University, 13(1), 173 - 189. https://so03.tci-thaijo.org/index.php/jhsc/article/view/244912
Manietta, C., Rommerskirch-Manietta, M., Purwins, D., & Roes, M. (2022). Consulting concepts and structures for people with dementia in Germany: a protocol for a 'grey-shaded' scoping review. BMJ Open, 12(4), e059771. https://doi.org/10.1136/bmjopen-2021-059771
Matichon. (2015, 8 August). Opposition to the Procurement Act: NGOs reveal it helps pharmaceutical companies to topple the Government Pharmaceutical Organization. Matichon, 7
Miles, M. B., Huberman, A. M., & Saldana, J. (2014). Qualitative data analysis: a methods sourcebook (Third ed.). SAGE Publications Ltd (CA).
National Drug Policy Development Committee. (2023). National Security Policy and Plan B.E. 2566 – 2570 (2023 - 2027). Government Gazette, 140(21A), 1.
National Health Notification on the statute on national health system No.3. (2023). Government Gazette, 140(84D), 13.
PATH (2011). An assessment of vaccine supply chain and logistics systems in Thailand. Author.
Sarnola, K., Ahonen, R., Martikainen, J. E., & Timonen, J. (2018). Policies and availability of orphan medicines in outpatient care in 24 European countries. European Journal of Clinical Pharmacology, 74(7), 895 - 902. https://doi.org/10.1007/s00228-018-2457-x
Sermsuk;, P., Pongsuwan;, W., Pongsuwan;, H., Hanpanich;, P., Techayuenyong;, T., Chuboonsub;, P., Tisyangkul;, R., Sansaporn;, S., Anukoolprasert;, P., & Namwan;, R. (2021). The efficient of procurement and distribution processes for Central Medicines Procurement in UC Scheme. Health Systems Research Institute. https://kb.hsri.or.th/dspace/handle/11228/5307
Siamrath. (2007, 31 October). Urge the government to review the drug procurement law. Siamrath, 3
Sonsuphap, R., Jesdapipat, S., & Phaksipaeng, I. (2022). Management of medical supplies for Coronavirus Disease 2019: problems, obstacles and solutions for public and private sector procurement. Health Systems Research Institute. https://kb.hsri.or.th/dspace/handle/11228/ 5556
Taratai, K., Kittiboonyakun, P., Sripong, P., Senalad, R., Akaleephan, C., & Pianchana, C. (2024). Management of medicines medical supplies, and vaccines during the COVID-19 pandemic: a case study from Maha Sarakham, Thailand. Mahasarakham Hospital Journal, 21(2), 166-178. https://he02.tci-thaijo.org/index.php/MKHJ/article/view/269498/184456
Thanitcul, S., & Braslow, M. L. (2013). Compulsory licensing of chronic disease pharmaceuticals in Thailand Thai Journal of Pharmaceutical Sciences, 37(2), 61 - 83. https://www.thaiscience. info/journals/Article/TJPS/10898711.pdf
Townsend, B. (2021). Defending access to medicines in regional trade agreements: lessons from the Regional Comprehensive Economic Partnership – a qualitative study of policy actors’ views. Globalization and Health, 17(1), 78. https://doi.org/10.1186/s12992-021-00721-4
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., … Straus, S. E. (2018). PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med, 169(7), 467 - 473. https://doi.org/10.7326/m18-0850
United Nations Development Programme. (2022). Using competition law to promote access to health technologies: a supplement to the guidebook for low- and middle-income countries https://www.undp.org/sites/g/files/zskgke326/files/2022-03/UNDP-Using-Competition-Law-to-Promote-Access-to-Health-Technologies.pdf
United Nations Thailand. (2024). Sustainable Development Goal 3 Good Health and Well-being. https://thailand.un.org/en/sdgs/3
Usavakidviree, V., Sooksriwong, C.-o., Chanjaruporn, F., Sermsinsiri, V., & Tosanguan, K. (2019). The reform of Thailand national security of drugs and medical supplies. Health Systems Research Institute. https://kb.hsri.or.th/dspace/handle/11228/5049
Watcharadamrongkun, A. (2018). Decision factors to purchase pharmaceutical ingredients for pharmaceutical industry. Journal of Management Science, Ubon Ratchathani University, 7(14), 53 - 66. https://so03.tci-thaijo.org/index.php/jms_ubu/article/view/164270
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Princess of Naradhiwas University Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.



