A New Yeast Strain, Nakaseomyces glabratus DL5, Isolated from a Mangrove Forest in Chonburi Province and Its Potential for Lipid Production
Keywords:
oleaginous yeasts, isolation of yeasts, mangrove forest, lipid productionAbstract
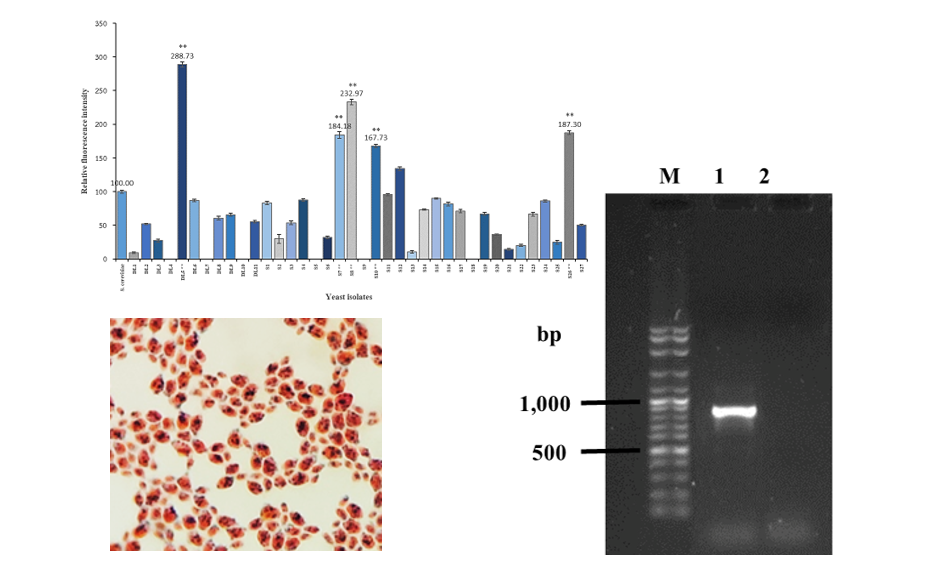
This research aimed to search for oleaginous yeasts from mangrove forest resources and primarily investigated their potentials for lipid production. Ten of soil and decaying leaf samples from the Nature Education Center for Mangrove Conservation in Chonburi province were collected and used for the isolation of yeasts on Yeast extract peptone dextrose medium. A total of 38 yeast strains were isolated in which 11 yeast isolates (DL1-DL11) and 27 yeast isolates (S1-S27) were obtained from decaying leaf and soil samples, respectively. These yeast isolates were screened for their high lipid accumulation by Nile red fluorescence assay. Of 38 yeast isolates, five isolates, DL5, S7, S8, S10 and S2, showed the possibility of being oleaginous strains. Those five isolates of yeast were selected based on their potentials of lipid accumulation and further visualized for their ability to accumulate intracellular lipid by Sudan Black B staining. Results showed that isolate DL5 obtained from the decaying leaf sample had the most distinct intracellular lipid accumulation in comparison with the other isolates. Identification of isolate DL5 was found to be Nakaseomyces glabratus on the basis of ITS1- 5.8S rDNA - ITS2 sequence. Further investigation of lipid production by N. glabratus DL5 showed that this strain achieved a lipid content of 9.61 ± 2.17 % of dry biomass weight at 120 hours of growth in DMY broth. These results are important preliminary data for further optimization to improve lipid production and accumulation in the yeast for future applications.
References
Ageitos, J.M., Vallejo, J.A., Veiga-Crespo, P. and Villa, T.G. 2011. Oily yeasts as oleaginous cell factories. Applied Microbiology and Biotechnology 90(4): 1219-1227.
Amornrattanapan, P. and Thongthep, P. 2019. Isolation and screening of oleaginous yeasts capable of using glycerol as a carbon source. Ramkhamhaeng Research Journal of Sciences and Technology 22(2): 61-70.
Berikten, D., Hoşgün, E.Z., Gökdal Otuzbiroğlu, A., Bozan, B. and Kıvanç, M. 2021. Lipid production from crude glycerol by newly isolated oleaginous yeasts: Strain selection, molecular identification and fatty acid analysis. Waste and Biomass Valorization 12: 5461-5470.
Burdon, K.L. 1946. Fatty material in bacteria and fungi revealed by staining dried and fixed slide preparations. Journal of Bacteriology 52(6): 665-678.
Castanha, R.F., Morais, L.A.S., Mariano, A.P. and Monteiro, R.T.R. 2013. Comparison of two lipid extraction methods production methods produced by yeast in cheese whey. Brazilian Archives of Biology and Technology 56(4): 629-636.
Chang, Y.H., Chang, K.S., Hsu, C.L., Chuang, L.T., Chen, C.Y., Huang, F.U. and Jang, H.D. 2013. A comparative study on batch and fed-batch cultures of oleaginous yeast Cryptococcus sp. in glucose-based media and corncob hydrolysate for microbial oil production. Fuel 105(1): 711-717.
Chanklan, R., Kungkaew, P., Am-In, S. and Jindamorakot, S. 2012. Diversity of yeasts in the Nature Education Center for mangrove Conservation and Ecotourism, Chonburi Province. Thai Journal of Science and Technology 1(3): 155-168. (in Thai)
Chomchuen, S., Arkornnak, M. and Amornrattanapan, P. 2021. Isolation and identification of oleaginous yeasts from soils at Bang Phra Reservoir, Chonburi province. Burapha Science Journal 26(2): 886-906. (in Thai)
Department of Alternative Energy Development and Efficiency. 2023. Energy Situation in Thailand from January to May 2023. Available Source: https://kc.dede.go.th/knowledge-view.aspx?p=478, September 20, 2023. (in Thai)
Dourou, M., Aggeli, D., Papanikolaou, S. and Aggelis, G. 2018. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Applied Microbiology and Biotechnology 102(6): 2509-2523.
Fakankun, I., Mirzaei, M. and Levin, D.B. 2019. Impact of culture conditions on neutral lipid production by oleaginous yeast, pp. 311-325. In Balan, V., eds. Microbial Lipid Production. Methods in Molecular Biology, Vol. 1995. Humana, New York.
Gabaldón, T. and Fairhead, C. 2018. Genomes shed light on the secret life of Candida glabrata: not so asexual, not so commensal. Current Genetics 65(1): 93-98.
Hall, T. 2013. BioEdit V7.2. Available Source: https://bioedit.software.informer.com/versions/, February 10, 2022.
Hoondee, P., Wattanagonniyom, T., Weeraphan, T., Tanasupawat, S. and Savarajara, A. 2019. Occurrence of oleaginous yeast from mangrove forest in Thailand. World Journal of Microbiology and Biotechnology 35(7): 108.
IEA. 2023. World Energy Investment 2023. Available Source: https://www.iea.org/reports/world-energy-investment-2023, September 20, 2023.
Jaingam, S., Sanevas, N. and Sirikhachornkit, A. 2016. Lipid production of a yeast strain isolated from a mangrove forest in Thailand, pp. 553-557. In The Proceedings of the 5th Marine Science Conference 2016. Bangkok. (in Thai)
Jape, A., Harsulkar, A. and Sapre, V.R. 2014. Modified Sudan Black B staining method for rapid screening of oleaginous marine yeasts. International Journal of Current Microbiology and Applied Sciences 3(9): 41-46.
Kolouchová, I., Schreiberová, O., Sigler, K., Masák, J. and Řezanka, T. 2015. Biotransformation of volatile fatty acids by oleaginous and non-oleaginous yeast species. FEMS Yeast Research 15(7): fov076.
Kumar, D., Singh, B. and Korstad, J. 2017. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renewable and Sustainable Energy Reviews 73: 654-671.
Kumar, K., Askari, F., Sahu, M. and Kaur R. 2019. Candida glabrata: A lot more than meets the eye. Microorganisms 7(2): 39.
Lee, N.L.Y., Huang, D., Quek, Z.B.R., Lee, J.N. and Wainwright, B.J. 2020. Distinct fungal communities associated with different organs of the mangrove Sonneratia alba in the Malay Peninsula. IMA Fungus 11: 17.
Limtong, S. 2006. Yeasts: Diversity and Biotechnology. Kasetsart University Press, Bangkok. (in Thai)
Miranda, C., Bettencourt, S., Pozdniakova, T., Pereira, J., Sampaio, P., Franco-Duarte, R. and Pais, C. 2020. Modified high-throughput Nile red fluorescence assay for the rapid screening of oleaginous yeasts using acetic acid as carbon source. BMC Microbiology 20: 60.
Mokobi, F. 2022. Sudan Black B staining. Available Source: https://microbenotes.com/sudan-black-b-staining/, October 28, 2022.
Müller-Langer, F., Majer, S. and O'Keeffe, S. 2014. Benchmarking biofuels - a comparison of technical, economic and environmental indicators. Energy Sustainability and Society 4: 20.
Pankin, K.E., Ivanova, Y.V., Kuz’mina, R.I. and Shtykov, S.N. 2011. Comparison of the physicochemical characteristics of biofuels and petroleum fuels. Chemistry and Technology of Fuels and Oils 47: 7-11.
Papanikolaou, S., Chevalot, I., Komaits, M., Marc, I. and Aggelis, G. 2001. Single cell oil production by Yarrowia lipolytica growing growing on an industrial derivative of animal fat in batch cultures. Applied Microbiology and Biotechnology 58(3): 308-312.
Prasatsri, K. 2006. Identification of yeast isolated from organic matters in mangrove forests by conventional and molecular taxonomy. Master of Science (Microbiology), Kasetsart University. (in Thai)
Ratledge, C. 1997. Microbial Lipids, pp. 133-197. In Rehm, H.J., Reed, R., Pulher, A., Stadler, P., Kleinkauf, H. and von Dohren, H., eds. Biotechnology: Products of Secondary Metabolism. Wiley-VCH, Weinheim.
Ratledge, C. 2001. Regulation of lipid accumulation in oleaginous microorganisms. Biochemical Society Transactions 30: 1047-1050.
Saran, S., Mathur, A., Dalal, J. and Saxena, R.K. 2017. Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel 188: 324-331.
Satyanarayana, T. and Kunze, G. 2009. Yeast biotechnology: Diversity and applications (1st ed). Springer, Dordrecht.
Schulze, I., Hansen, S., Großhans, S., Rudszuck, T., Ochsenreither, K., Syldatk, C. and Neumann, A. 2014. Characterization of newly isolated oleaginous yeasts - Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express 4: 24.
Sha, Q. 2013. A comparative study on four oleaginous yeasts on their lipid accumulating capacity. Master of Science (Microbiology), Swedish University of Agricultural Science.
Sitepu, I.R., Ignatia, L., Franz, A.K., Wong, D.M., Faulina, S.A., Tsui, M., Kanti, A. and Mills, K.B. 2012. An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. Journal of Microbiological Methods 91: 321-328.
Sriwongchai, S., Prasongsuk, S., Leerat, N. and Kitleartpornpairoat, R. 2018. Possibility of soil mangrove-derived oleaginous yeast Rhodotorula mucilaginosa on nutritionally optimized medium for lipid production for alternative biodiesel feedstock. Burapha Science Journal 23(1): 304-317. (in Thai)
Tapia, E.V., Anschau, A.A., Coradini, A.L., Franco, T.T. and Deckmann, A.C. 2012. Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express 2: 64.
Thangavelu, K., Sundararaju, P., Srinivasan, N. and Uthandi, S. 2021. Bioconversion of sago processing wastewater into biodiesel: Optimization of lipid production by an oleaginous yeast, Candida tropicalis ASY2 and its transesterification process using response surface methodology. Microbial Cell Factories 20: 167.
White, T.J., Bruns, T., Lee, S.J.W.T. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315 - 322. In Innis, M.A., Gelfand, D.H., Sninsky, J.J. and White, T.J., eds. PCR protocols: A Guide to Methods and Applications. Academic Press, New York.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Recent Science and Technology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The content and information in the article published in Journal of Rajamangala University of Technology Srivijaya It is the opinion and responsibility of the author of the article. The editorial journals do not need to agree. Or share any responsibility.






