Optimization of minerals and plant growth regulators for micropropagation of strawberry ‘Pharachatan 80’
Keywords:
Micropropagation, mineral nutrition, response surface methodology, strawberry ‘Pharachatan 80’Abstract
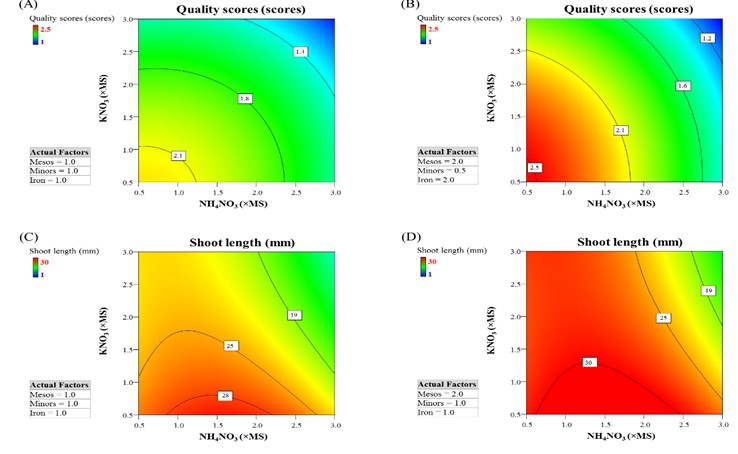
Micropropagation is important for rapid multiplication of a wide range of nursery crops, including strawberry. Although most plant species or cultivars can grow on Murashige and Skoog (MS) medium, some display non-optimal growth. This study used response surface methodology (RSM) to study the effect of MS minerals on micropropagated strawberry growth and determine which of these minerals are critical for improving growth. In vitro growth of strawberry ‘Pharachatan 80’ was determined by varying five factors that included NH4NO3, KNO3, mesos salts (CaCl2, KH2PO4 and MgSO4), minor elements, and EDTA-chelated iron. The effects of these five factors on plant quality, multiplication, shoot length, and leaf color were determined and validated. The results showed that modified MS as 1.0×NH4NO3, 0.5×KNO3, 2.0×Mesos, 3.0× Minors and 2.0×Iron significantly improved growth of strawberry ‘Pharachatan 80’. Finally, modified MS supplemented with 1 mg/L BAP was suitable for shoot multiplication.
References
Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006; 54:9329-9339.
Epstein E. editor. Mineral nutrition of plants: Principles and Perspective, 1st ed. London: John Wiley and Sons Inc; 1972. P. 412.
George EF, Hall MA, De Klerk GJ. The components of plant tissue culture media I: macro- and micro-nutrients, In: George EF, Hall MA, De Klerk GJ, editors. Plant Propagation by Tissue Culture, 3rd ed. New York: Springer; 2008. P. 65-113.
Hildebrandt AC, Riker A, Duggar B. The influence of the composition of the medium on growth in vitro of excised tobacco and sunflower tissue cultures. Am J Bot. 1946;33(7):591-597.
Driver JA, Kuniyuki AH. In vitro propagation of Paradox walnut rootstock. HortSci. 1984;19:507-509.
Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151-158.
Lloyd G, McCown B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Combined Proceedings, International Plant Propagators' Society. 1980;30:421-427.
Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473-497.
Niedz RP, Evens TJ. Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol-Plant. 2007;43:370-381.
Reed BM, Wada S, DeNoma J, Niedz RP. Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol-Plant. 2013;49:343-355.
Wada S, Niedz RP, DeNoma J, Reed BM. Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cell Dev Biol-Plant. 2013;49:356-365.
Poothong S, Reed BM. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci Hortic. 2014;165:132-41.
Poothong S, Reed BM. Increased CaCl2, MgSO4, and KH2PO4 improve the growth of micropropagated red raspberries. In Vitro Cell Dev Biol-Plant. 2015;51(6):648-658.
Aranda-Peres AN, Martinelli AP, Pereira-Peres LE, Higashi EN. Adjustment of mineral elements in the culture medium for the micropropagation of three Vriesea bromeliads from the Brazilian Atlantic Forest: The importance of calcium. Hort Sci. 2009;44(1);106-112.
Hand CR, Maki S, Reed BM. Modeling optimal mineral nutrition for hazelnut (Corylus avellana) micropropagation. Plant Cell Tiss Organ Cult. 2014;119:411-425.
El-Hawaz R, Park D, Bridges WC, Adelberg J. Optimizing in vitro mineral nutrition and plant density increases greenhouse growth of Curcuma longa L. during acclimatization. Plant Cell Tiss Organ Cult. 2016;126:33-42.https://doi.org/10.1007/s11240-016-0974-9.
Greenway MB, Phillips IC, Lloyd MN, Hubstenberger JF, Phillips GC. A nutrient medium for diverse applications and tissue growth of plant species in vitro. In Vitro Cell Dev Biol-Plant. 2012; 48:403-410.
Gonçalves S, Correia PJ, Martins-Loução, MA, Romano A. A new medium formulation for in vitro rooting of carob tree based on leaf macronutrients concentrations. Biol Plant. 2005;49(2), 277–280. https://doi.org/10.1007/s10535-005-7280-4.
Bouman H, Tiekstra A. Adaptions of the mineral composition of tissue culture media on the basis of plant elemental analysis and composition of hydroponic substrates, In: Hvoslef-Eide AK, Preil W, editors. Liquid Culture Systems for in vitro Plant Propagation. Springer, Dordrecht. 2005;493-505.
Sakila S, Ahmed M, Roy U, Biswas M, Karim R, Razvy M, et al. Micropropagation of strawberry (Fragaria x ananassa Duch.) a newly introduced crop in Bangladesh. Am-Eurasian J Sci Res. 2007;2(2):151-154.
Ashrafuzzaman M, Faisal S, Yadav D, Khanam D, Raihan F. Micropropagation of strawberry (Fragaria ananassa) through runner culture. Bangladesh J Agri Res. 2013;38(3):467-472.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Naresuan Phayao Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







