Fabrication of graphene-gold nanoparticles hybrid materials as active catalysts for the degradation of formaldehyde
Keywords:
Hybrid materials, Graphene, Gold nanoparticle, Catalyst, FormaldehydeAbstract
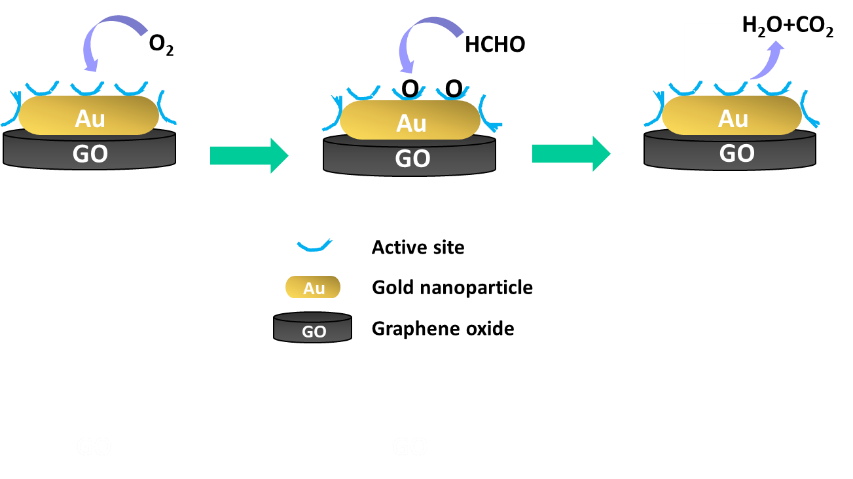
This research studies the fabrication of graphene /gold nanoparticles (AuNPs) hybrid materials by a facile method. To incorporate AuNPs on to graphene, graphene colloidal solution and gold chloride tetrahydrate with various concentration of 1, 4, 8, 40 and 80 weight percent were mixed and stirred overnight at ambient temperature for 24 hours and further reduced with Sodium borohydride. To study the catalytic activity of graphene / AuNPs hybrid materials, the obtained products were subjected to test for the formaldehyde degradation using UV-visible spectroscopy. The study illustrates that graphene / AuNPs hybrid material (8 wt% AuNPs) at pH 10 shows catalytic activities for formaldehyde degradation around 36 percent while the catalytic activities of graphene / AuNPs hybrid materials with 40 and 80 wt% of AuNPs reach more than 80 percent. The obtained products of graphene / AuNPs hybrid materials could be applied as catalyst for the degradation of Formaldehyde in solutions / fluids.
References
Abraham S, König M, Srivastava SK, Kumar V, Walkenfort B, Srivastava A. A Carbon nanostructure (0-3 dimensional) supported isolated gold nanoparticles as an effective SERS substrate. Sens. Actuators 2018;273:455-465.
Ayati A, Ahmadpour A, Bamoharram FF, Tanhaei B, Mänttärid M, Sillanpää M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014;107:163–174.
Chen FC, Chuang MK, Hsu CS. Gold nanoparticles-graphene oxide nanocomposites that enhance
the device performance of polymer solar cells. Journal of Nanomaterials 2014. Article ID 736879.
Available from https://doi.org/10.1155/2014/736879.
Choi HC, Park Y, Koo JY, Kim S, Choi HC. Spontaneous formation of gold nanoparticles on graphene by galvanic reaction through graphene. ASC Omega 2019;4:18423-18427.
Choucair M, Thordarson P, Stride JA. Gram-scale production of grapheme based on Solvothermal synthesis and sonication. Nat Nano 2009; 4(1):30-33.
Hummer WS, Offeman RE. Preparation of graphite oxide. J Am Chem Soc 1998;80:1339.
Lerf A, He H Y, Forster M, Klinowski J. Structure of graphite oxide revisited. J. Phys. Chem 1998;102:4477-4482.
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nature Nanotechnology 2008;3:101-105.
Lu J, Yang JX, Wang J, Lim A, Wang S, Loh KP. One-Pot synthesis of fluorescent carbon nanoribbons, nanoparticle and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009;3(8):2367-2375.
Nepal D, Ren Y, Rao R, Bhusal S, Varshney V, Kedziora G, Wheeler R, Kang Y, Roy A. Hierarchical assembly of gold nanoparticles on graphene nanoplatelets by spontaneous reduction: Implications for smart composites and biosensing. ASC Appl. Nano Mater 2020; 3:8753-8762.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV. Electric field effect in atomically thin carbon films. Science 2004;306(5696):666-669.
Pandy PC, Shukla S, Pandy Y. 3-Aminopropyltrimethoxysilane and graphene oxide/reduced graphene oxide-induced generation of gold nanoparticles and their nanocomposites: electrocatalytic and kinetic activity. RSC Adv 2016;6(84).
Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat Nano 2009;4(4):217-224.
Sirajuddin, Mechler A, Torriero AAJ, Nafady A, Lee CY, Bond AM, Mullance APO, Bhargava SK. The formation of gold nanoparticles using hydroquinone as a reducing agent through a localized pH change upon addition of NaOH to a solution of HAuCl4. Colloids and Surfaces 2010;370:35-41.
Movahed SK, Fakharian M, Dabiri M, Bazgir A. Gold nanoparticle decorated reduced graphene oxide sheets with high catalytic activity for Ullmann homocoupling. RSC Adv 2014;4(10):5243-5247.
Zhu X, Cheng B, Yu J, Ho W. Halogen poisoning effect of Pt-TiO2 for formaldehyde catalytic oxidation performance at room temperature. Applied Surface Science 2016;364:808-814.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 University of Phayao

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยาไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย
The authors are themselves responsible for their contents. Signed articles may not always reflect the opinion of University of Phayao. The articles can be reproduced and reprinted, provided that permission is given by the authors and acknowledgement must be given.







