การยับยั้งการเจริญของจุลินทรีย์ก่อโรคและการเป็นพิษต่อเซลล์มะเร็ง ด้วยสารสกัดจากต้นโทงเทง
Main Article Content
บทคัดย่อ
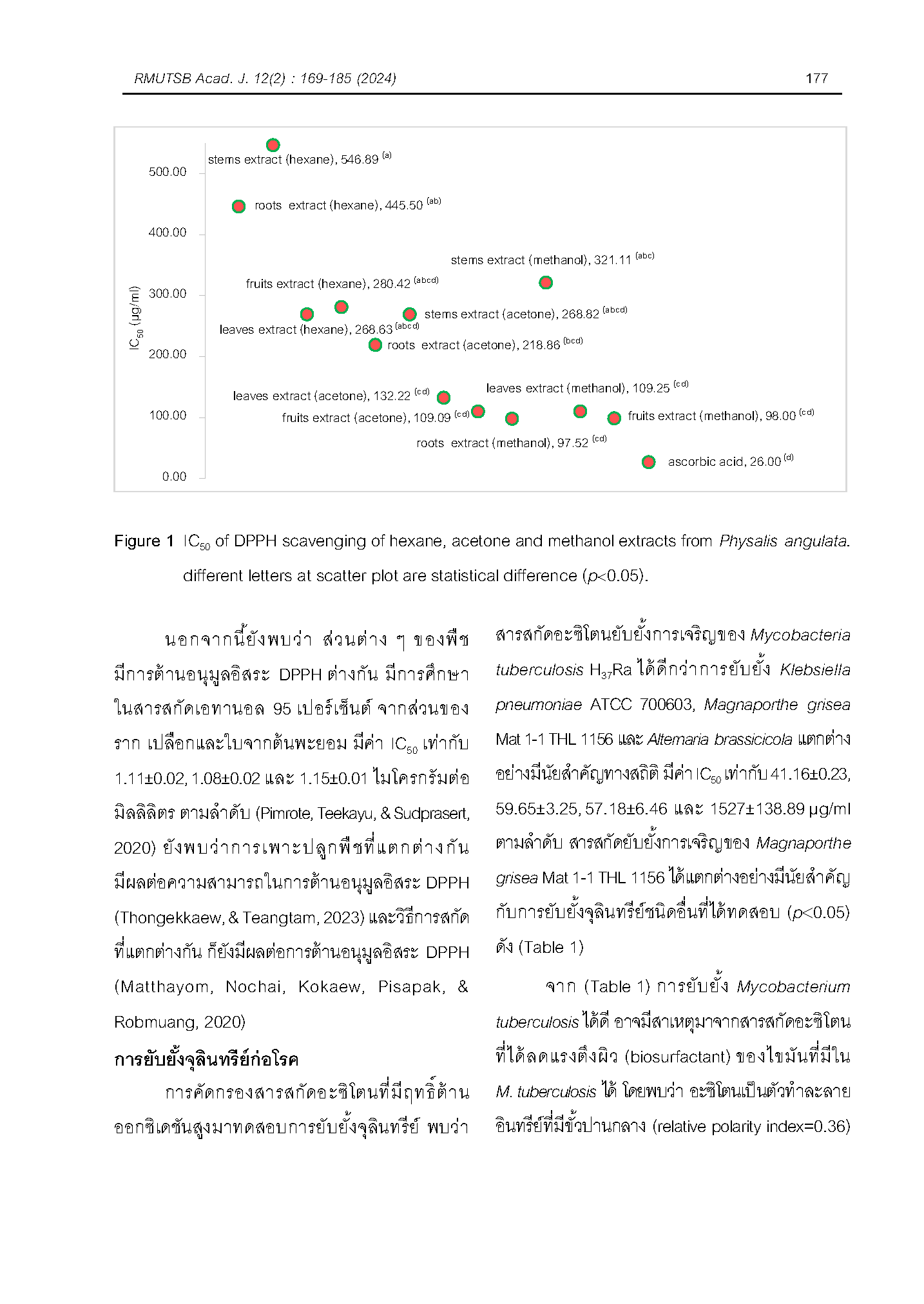
การศึกษาสารสกัดจากต้นโทงเทง (Physalis angulata) ด้วยเครื่องอัลตราโซนิกความถี่ 45 กิโลเฮิรตซ์ ที่อุณหภูมิ 40 องศาเซลเซียส นาน 30 นาที พบว่า ให้ผลการต้าน DPPH การต้านแบคทีเรียก่อโรค 4 ชนิด คือ Mycobacterium tuberculosis, Klebsiella pneumoniae, Magnaporthe grisea และ Alternaria brassicicola รวมถึงการทดสอบการต้านเซลล์มะเร็งในหลอดทดลอง 3 ชนิด คือ เซลล์มะเร็งปอด (NCI-H187) เซลล์มะเร็งตับ (HepG2) เซลล์มะเร็งช่องปาก (KB) และเซลล์มะเร็งลำไส้ใหญ่ (Caco2) จากการทดสอบพบว่า ค่า IC50 การต้านอนุมูลอิสระ DPPH สารสกัดเมทานอลจากราก ลำต้น ใบและผล สารสกัดอะซิโตนจากราก ใบและผล แตกต่างอย่างไม่มีนัยสำคัญกับ ค่า IC50 ของกรดแอสคอร์บิก (p³0.05) มีค่า 97.52±22.29, 321.11±160.79, 109.25±7.76, 98.00±3.93, 218.86±55.25, 132.22±7.63, 109.09±7.45 และ 26.00±0.36 µg/ml ตามลำดับ สารสกัดเฮกเซนจากราก มีค่า IC50 สูงสุด แตกต่างอย่างมีนัยสำคัญทางสถิติกับสารสกัดอะซิโตนและเมทานอล มีค่า IC50 เท่ากับ 546.89±216.91 µg/ml พบว่า สารสกัดอะซิโตนจากผลยับยั้งการเจริญของเชื้อ M. tuberculosis ได้ดีกว่าเชื้อ K. pneumoniae เชื้อ M. grisea และเชื้อ A. brassicicola อย่างมีนัยสำคัญ และนอกจากนี้สารสกัดดังกล่าวยังมีความเป็นพิษต่อเซลล์มะเร็งปอดซึ่งมีความแตกต่างจากเซลล์มะเร็งตับ เซลล์มะเร็งช่องปาก และเซลล์มะเร็งลำไส้ใหญ่อย่างมีนัยสำคัญ (p<0.05) อีกด้วย แนวทางการใช้ประโยชน์จากการศึกษานี้ เพื่อส่งเสริมการใช้สมุนไพรเพื่อลดไขมันสะสมเกินความจำเป็น
Article Details

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ต้นฉบับที่ได้รับการตีพิมพ์ถือเป็นสิทธิของเจ้าของต้นฉบับและของวารสารวิชาการ มทร.สุวรรณภูมิ เนื้อหาบทความในวารสารเป็นแนวคิดของผู้แต่ง มิใช่เป็นความคิดเห็นของคณะกรรมการการจัดทำวารสาร และมิใช่ความรับผิดชอบของมหาวิทยาลัยเทคโนโลยีราชมงคลสุวรรณภูมิ
เอกสารอ้างอิง
Barceló-Coblijn, G., Martin, M. L., De Almeida, R. F. M., Noguera-Salvà, M. A., Marcilla-Etxenike, A., Guardiola Serrano, F., Lüth, A., Kleuser, B., Halver, J. E., & Escribá, P. V. (2011). Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proceedings of the National Academy of Sciences of the United States of America, 108(49), 19569-19574.
Belayneh, Y. M., Birhanu, Z., Birru, E. M., & Getenet, G. (2019). Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae). Journal of Experimental Pharmacology, 11(1), 29-38.
Beveridge, T. J. (1990). Mechanism of gram variability in select bacteria. Journal of bacteriology, 172(3), 1609-1620.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199-1200.
Boonsombat, J., Chawengrum, P., Mahidol, C., Kittakoop, P., Ruchirawat, S., & Thongnest, S. (2020). A new 22, 26-seco physalin steroid from Physalis angulata. Natural Product Research, 34(8), 1097-1104.
Brennan, P. J., & Nikaido, H. (1995). The envelope of mycobacteria. Annual review of biochemistry, 64(1), 29-63.
Cabrera-Sanchez, J., Cuba, V., Vega, V., Van der Stuyft, P., & Otero, L. (2022). Lung cancer occurrence after an episode of tuberculosis: a systematic review and meta-analysis. European Respiratory Review, 31(165), 1-13.
Changsen, C., Franzblau, S. G., & Palittapongarnpim, P. (2003). Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrobial Agents and Chemotherapy, 47(12), 3682-3687.
Chooklin C. S., Chaichan, W., & Dikit P. (2021). The Potential of biosurfactant from pomelo peel fermentation for bacterial inhibition. Rajamangala University of Technology Srivijaya Research Journal, 13(3), 704-716. (in Thai)
Choosong, J., & Siriruk P. (2014). Development of the herbal soap product from the palmyrah fruit of the conservation tourism group, Sathingpra district, Songkhla province. RMUTSB Academic Journal, 2(2), 165-173. (in Thai)
Clinical and Laboratory Standards Institute (CLSI). (2002). Reference method for broth microdilution antifungal susceptibility testing of filamentous fungi; Approved standard. CLSI document M38-A, Wayne, Pennsylvania.
Clinical and Laboratory Standards Institute (CLSI). (2006a). Method for dilution antimicrobial susceptibility test for bacteria that growth aerobically; Approve standard 7th edition. CLSI document M7-A7 vol. 26, no. 2. Wayne, Pennsylvania.
Clinical and Laboratory Standards Institute (CLSI). (2006b). Performance standards for antimicrobial susceptibility testing; Sixteenth informational supplement. CLSI document M100-S16, vol. 26, no. 3, Wayne, Pennsylvania.
Collins, L. A., Torrero, M. N., & Franzblau, S. G. (1998). Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy, 42(2), 344-347.
Cornell College of Agriculture and Life Sciences (Cornell CALS). (2019). Saponins. Retrieved 10 January 2024, from https://poisonousplants.ansci.cornell.edu/toxicagents/saponin.html
Cukic, V. (2017). The association between lung carcinoma and tuberculosis. Medical Archives, 71(3), 212-214.
De Oliveira, A. M., Malunga, L. N., Perussello, C. A., Beta, T., & Ribani, R. H. (2020). Phenolic acids from fruits of Physalis angulata L. in two stages of maturation. South African Journal of Botany, 131(1), 448-453.
Dougnon, T. V., Bankolé, H. S., Klotoé, J. R., Sènou, M., Fah, L., Koudokpon, H., Akpovi, C., Dougnon T. J., Addo, P. Loko, F., & Boko, M. (2014). Treatment of hypercholesterolemia: screening of Solanum macrocarpon Linn (Solanaceae) as a medicinal plant in Benin. Avicenna Journal of Phytomedicine, 4(3), 160-169.
Ferreira, L. M. D. S. L., Vale, A. E. D., Souza, A. J. D., Leite, K. B., Sacramento, C., Moreno, M. L. V., Araujo, T. H., Soares, M. B. P., & Grassi, M. F. R. (2019). Anatomical and phytochemical characterization of Physalis angulata L.: a plant with therapeutic potential. Pharmacognosy Research, 11(2), 171-177.
Forest Herbarium. (2023). Physalis angulata L. Retrieved 15 January 2023, from https://www.dnp.go.th/botany/mindexdictdetail.aspx?runno=2832 (in Thai)
Guarro, J., Pujol, I., Aguilar, C., Llop, C., & Fernandez-Ballart, J. (1998). Inoculum preparation for in-vitro susceptibility testing of filamentous fungi. The Journal of Antimicrobial Chemotherapy, 42(3), 385-387.
Haugland, R. P. (2002). Assay for cell viability, proliferation and function. Handbook of Fluorescent Probes and Research Products. Eugene, Oregon: Molecular Probes.
Hseu, Y. C., Wu, C. R., Chang, H. W., Senthil-Kumar, K. J., Lin, M. K., Chen, C. S., Cho, H. J., Huang, C. Y., Huang, C. Y., Lee, H. Z., Hsieh, W. T., Chung, J. G., Wang, H. M., & Yang, H. L. (2011). Inhibitory effects of Physalis angulata on tumor metastasis and angiogenesis. Journal of Ethnopharmacology, 135(3), 762-771.
Kor-arnan, S., Paoblake, S., & Aswachaisuvikom, T. (2015). Antibacterial, antioxidation, antiproteolytic, and cytotoxicity activity of Stevia rebaudiana Bertoni Leaves. Journal of Science and Technology, Ubon Ratchathani University, 17(3), 48-55. (in Thai)
Korchowiec, B., Gorczyca, M., Wojszko, K., Janikowska, M., Henry, M., & Rogalska, E. (2015). Impact of two different saponins on the organization of model lipid membranes. Biochimica et Biophysica Acta Biomembranes, 1848(10), part A, 1963-1973.
Krishna, T. M., Vadluri, R., & Kumar, E. M. (2013). In vitro determination of antioxidant activity of Physalis angulata Lnn. International Journal of Pharma and Bio Sciences, 4(3), 541-549.
Kumagai, M., Yoshida, I., Mishima, T., Ide, M., Fujita, K., Doe, M., Nishikawa, K., & Morimoto, Y. (2021). 4β-Hydroxywithanolide E and withanolide E from Physalis peruviana L. inhibit adipocyte differentiation of 3T3-L1 cells through modulation of mitotic clonal expansion. Journal of Natural Medicines, 75(1), 232-239.
Kusumaningtyas, R. W., Laily, N., & Limandha, P. (2015). Potential of ciplukan (Physalis angulata L.) as source of functional ingredient. Procedia Chemistry, 14(1), 367-372.
Lee, H., Woo, S. M., Jang, H., Kang, M., & Kim, S. Y. (2022). Cancer depends on fatty acids for ATP production: a possible link between cancer and obesity. Seminars in Cancer Biology, 86(2), 347-357.
Maldonado, E., Hurtado, N. E., Pérez-Castorena, A. L., & Martínez, M. (2015). Cytotoxic 20, 24-epoxywithanolides from Physalis angulata. Steroids, 104(1), 72-78.
Malik, I., Csollei, J., Jampílek, J., Stanzel, L., Zadrazilova, I., Hosek, J., Pospísilova, S., Cízek, A., Coffey, A., & O'Mahony, J. (2016). The structure-antimicrobial activity relationships of a promising class of the compounds containing the N-arylpiperazine scaffold. Molecules, 21(10), 1274.
Matthayom, W., Nochai, K., Kokaew, K., Pisapak, K., & Robmuang, D. (2020). Investigation of antioxidant capacity and antioxidant activity of Ma-huad (Lepisanthes rubiginosa (Roxb.) Leenh.) juice. RMUTSB Academic Journal, 8(2), 187-198. (in Thai)
Meira, C. S., Guimarães, E. T., Dos Santos, J. A., Moreira, D. R., Nogueira, R. C., Tomassini, T. C., Ribeiro, I. M., De Souza, C. V., Ribeiro Dos Santos, R., & Soares. M. B. (2015). In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine, 22(11), 969-974.
National Institutes of Health (NIH). (2023). Lipid Storage Diseases. Retrieved 27 December 2023, from https://www.ninds.nih.gov/health-information/disorders/lipid-storage-diseases
National Library of Medicine (NLM). (2023). Bacillus cereus. Retrieved 27 December 2023, from https://www.ncbi.nlm.nih.gov/books/NBK459121/
Neto, R. N. M., de Barros Gomes, E., Weba-Soares, L., Dias, L. R. L., da Silva, L. C. N., & de Miranda, R. C. M. (2019). Biotechnological production of statins: metabolic aspects and genetic approaches. Current pharmaceutical biotechnology, 20(15), 1244-1259.
O'Brien, J., Wilson, I., Orton, T., & Pognan, F. (2000). Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry, 267(17), 5421-5426.
Paradelo, R., Moldes, A. B., Dominguez, J. M., & Barral, M. T. (2009). Reduction of water repellence of hydrophobic plant substrates using biosurfactant produced from hydrolyzed grape marc. Journal of Agricultural and Food Chemistry, 57(11), 4895-4899.
Pietro, R. C., Kashima, S., Sato, D. N., Januário, A. H., & França, S. C. (2000). In vitro antimycobacterial activities of Physalis angulata L. Phytomedicine, 7(4), 335-338.
Pimrote, K., Teekayu, K., & Sudprasert, P. (2020). Antioxidant activity and inhibition effect on pseudomonas aeruginosa of extracts from Pa-Yom (Shorea roxburghii G. Don). RMUTSB Academic Journal, 8(1), 15-27. (in Thai)
Pinto, N. B., Morais, T. C., Carvalho, K. M. B., Silva, C. R., Andrade, G. M., Brito, G. A. C., Veras, M. L., Pessoa, O. D. L., Rao, V. S., & Santos, F. A. (2010). Topical anti-inflammatory potential of Physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine, 17(10), 740-743.
Pourmasoumi, M., Hadi, A., Rafie, N., Najafgholizadeh, A., Mohammadi, H., & Rouhani, M. H. (2018). The effect of ginger supplementation on lipid profile: a systematic review and meta-analysis of clinical trials. Phytomedicine, 43(1), 28-36.
Reichardt, C. & Welton, T. (2011). Solvents and Solvent Effects in Organic Chemistry. Weinheim, Germany: Wiley‐VCH Verlag GmbH & Co. KGaA.
Reynolds, J., Moyes, R. B., & Breakwell, D. P. (2009). Differential staining of bacteria: acid fast stain. Current Protocols in Microbiology, Supplement 15, Appendix A3H, 1-5.
Rivera, D. E., Ocampo, Y. C., Castro, J. P., Caro, D., & Franco, L. A. (2015). Antibacterial activity of Physalis angulata L., Merremia umbellata L., and Cryptostegia grandiflora Roxb. Ex R. Br.-medicinal plants of the Colombian Northern Coast. Oriental Pharmacy and Experimental Medicine, 15, 95-102.
Row, L. R., Sarma, N. S., Matsuura, T., & Nakashima, R. (1978). Physalins E and H, new physalins from Physalis angulata and P. lancifolia. Phytochemistry, 17(9), 1641-1645.
Rouhi-Boroujeni, H., Rouhi-Boroujeni, H., Heidarian, E., Mohammadizadeh, F., & Rafieian-Kopaei, M. (2015). Herbs with anti-lipid effects and their interactions with statins as a chemical anti-hyperlipidemia group drugs: a systematic review. ARYA atherosclerosis, 11(4), 244-251.
Sala, A., Bordes, P., & Genevaux, P. (2014). Multiple toxin-antitoxin systems in Mycobacterium tuberculosis. Toxins, 6(3), 1002-1020.
Salamon, H., Bruiners, N., Lakehal, K., Shi, L., Ravi, J., Yamaguchi, K. D., Pine, R., & Gennaro, M. L. (2014). Cutting edge : vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. Journal of Immunology, 193(1), 30-34.
Shahzad, T., Ahmad, I., Choudhry, S., Saeed, M. K., & Khan, M. N. (2014). DPPH free radical scavenging activity of tomato, cherry tomato and watermelon: lycopene extraction, purification and quantification. International Journal of Pharmacy and Pharmaceutical Sciences, 6(suppl.2), 223-228.
Slayden, R. A., Dawson, C. C., & Cummings, J. E. (2018). Toxin-antitoxin systems and regulatory mechanisms in Mycobacterium tuberculosis. Pathogens and Disease, 76(4), 1-12.
Sun, C. P., Qiu, C. Y., Zhao, F., Kang, N., Chen, L. X., & Qiu, F. (2017). Physalins V-IX, 16, 24-cyclo-13, 14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Scientific reports, 7, 4057.
Tanner, L., Haynes, R. K., & Wiesner, L. (2020). Accumulation of TB-active compounds in murine organs relevant to infection by Mycobacterium tuberculosis. Frontiers in Pharmacology, 11, 724.
Thongekkaew, J., & Teangtam, W. (2023). Antioxidant activity, vitamin c and total phenolic contents in three types of lettuce grown in hydroponics and soil-based. RMUTSB Academic Journal, 11(2), 186-198. (in Thai)
World Health Organization (WHO). (2021a). Global tuberculosis report 2021. Retrieved 27 December 2023, from https://www.who.int/publications/i/item/9789240037021
World Health Organization (WHO). (2021b). Tuberculosis. Retrieved 27 December 2023, from https://www.who.int/news-room/fact-sheets/detail/tuberculosis
Xu, R., Yang, K., Li, S., Dai, M., & Chen, G. (2020). Effect of green tea consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials. Nutrition journal, 19, 48.
Xue, Y. W., Itoh, H., Dan, S., & Inoue, M. (2022). Gramicidin A accumulates in mitochondria, reduces ATP levels, induces mitophagy, and inhibits cancer cell growth. Chemical Science, 13(25), 7482-7491.
Yang, H., Geng, Y. H., Wang, P., Zhang, H. Q., Fang, W. G., & Tian, X. X. (2022). Extracellular ATP promotes breast cancer chemoresistance via HIF-1α signaling. Cell Death & Disease, 13, 199.
Yen, C. Y., Chiu, C. C., Chang, F. R., Chen, J. Y. F., Hwang, C. C., Hseu, Y. C., Yang, H. L., Lee, A. Y. L., Tsai, M. T., Guo, Z. L., Cheng, Y. S., Liu, Y. C., Lan, Y. H., Chang, Y. C., Ko, Y. C., Chang, H. W., & Wu, Y. C. (2010). 4β-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC Cancer, 10, 46.