ผลของอะดีนีนซัลเฟตและชนิดน้ำตาลต่อการเจริญและพัฒนาชักนำการเกิดยอดของแคลลัสมะละกอในสภาพปลอดเชื้อ
คำสำคัญ:
อะดีนีนซัลเฟต, น้ำตาล, แคลลัส, มะละกอ, ในสถาพปลอดเชื้อบทคัดย่อ
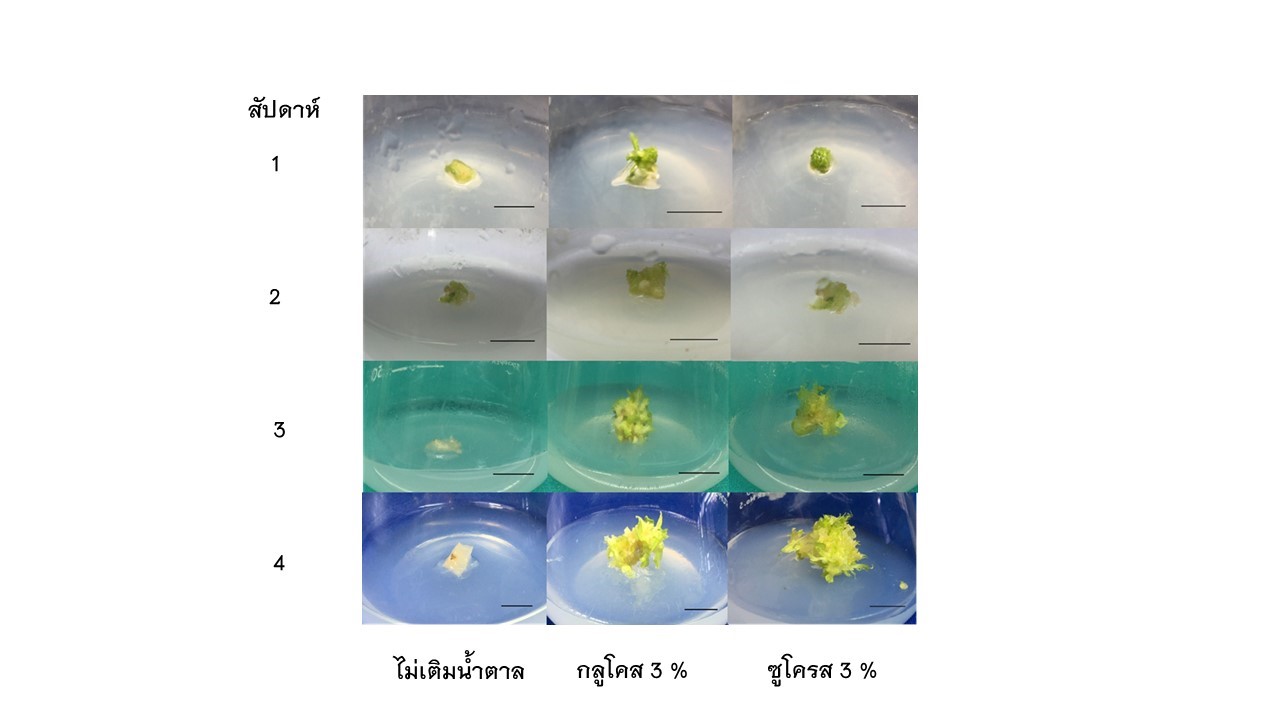
การศึกษานี้มีวัตถุประสงค์เพื่อศึกษาความเข้มข้นที่แตกต่างกันของอะดีนีนซัลเฟต (Adenine sulfate; Ads) และชนิดของน้ำตาลต่อการเจริญและพัฒนาการชักนำการเกิดยอดของชิ้นส่วนแคลลัสมะละกอ มี 2 การทดลอง โดยการทดลองที่ 1 การศึกษาระดับความเข้มข้นของ Ads ที่แตกต่างกัน คือ ไม่เติม (ชุดควบคุม), 25, 50 และ 75 มิลลิกรัมต่อลิตร เติมในอาหารสูตร Murashige and Skoog (MS) บันทึกผลหลังจากทำการเพาะเลี้ยงเป็นเวลา 4 สัปดาห์ จากผลการทดลองพบว่า ชิ้นส่วนแคลลัสที่เพาะเลี้ยงบนอาหารที่เติม Ads ความเข้มข้น 50 มิลลิกรัมต่อลิตร สามารถชักนำการเกิดยอดใหม่ได้มากที่สุด (7.00 ยอดต่อชิ้นส่วน) และความยาวของยอดใหม่มากที่สุด (5.15 มิลลิเมตร) ส่วนการทดลองที่ 2 การศึกษาชนิดของน้ำตาล 2 ชนิด คือ ซูโครสและกลูโคส เปรียบเทียบกับที่ไม่เติมน้ำตาล (ชุดควบคุม) พบว่าร้อยละการเกิดยอดใหม่และจำนวนยอดใหม่ต่อชิ้นส่วนไม่มีความแตกต่างเพื่อเพาะเลี้ยงบนอาหารที่เติมน้ำตาลทั้งสองชนิด แต่แตกต่างจากการที่ไม่เติมน้ำตาล (ชุดควบคุม) ถึงอย่างไรชิ้นส่วนแคลลัสที่เพาะเลี้ยงบนอาหารที่เติมกูลโคส
ร้อยละ 3 มีจำนวนยอดใหม่ต่อชิ้นส่วนเพิ่มขึ้น
เอกสารอ้างอิง
Office of Agricultural Economics. Exports of agricultural products and products of Thailand. In the report of Thai agricultural products trade with foreign countries for the year 2021. Office of Agricultural Economics 2022;1-75.
Yingyong P. Plant Propagation. 1thed. Bangkok: Baanlaesuan Publishing; 2019.
Penkhae R, Kiriya S and Kanjana L. Propagation of papaya (Carica papaya L.) cv. Holland in tissue culture. Khon Kaen AGR. J 2019;47(3):459-66.
Petcharat C. Plant tissue culture technology for agriculture. Bangkok: Bangkok Thonburi University;2023.
Phongyuth N, Aphichat C and Pitak P. Plant Propagation by Plant Tissue Culture Technique. Rajamangala Institute of Technology 2019;40 p.
Noguchi L, Uscharee R, Somkiat S and Somchai W. Effect of disinfectants and plant growth regulator in aquatic plant Bucephalandra sp. micropropagation. King Mongkut's Agricultural Journal 2017;35:95-103.
Afshan N, Anwar S, and Mohammad A. Effect of adenine sulphate interaction on growth and development of shoot regeneration and inhibition of shoot tip necrosis under In Vitro condition in adult Syzygium cumini L.—a multipurpose tree. Appl Biochem Biotechnol 2014;173:90–102.
Nancy J, Yashodhara V and Pragati M. Elicitation enhanced the production of bioactive compound and biomass accumulation in callus cultures of Glycyrrhiza glabra L. In Vitro. Cellular and Developmental Biology – Plant 2021;58:427–436.
Badou BT, Agbidinoukoun A, Cacai GTH, Dossoukpevi RC and Ahanhanzo C. Effects of system benzylaminopurine-adenine sulphate in combination with naphthalene acetic on in vitro regeneration and proliferation of pineapple (Ananas comosus (L.) Mill var. comosus). Int. J. Pure, Appl, Biosci 2017;9:1-15.
Siriwan B, Vanida C, and Supat A. Effects of plant growth regulators on papayas (Carica papaya) cultured in vitro. Kasetsart Journal (Natural Sciences) (Thailand) 1988;22(5):1-6.
Bonga JM, and Aderkas P. In vitro culture of trees. Dordrecht. Kluwer Academic Publishers, Chicago;1992;236 p.
Akhtar A, Zink D and Becker PB. Chromodomains are protein-RNA interaction modules. Nature 2000;407(6802):405-409.
Yudkin J, Edelman J and Hough L. Sugar: Chemical, Biological and Nutritional Aspects of Sucrose, Butterworth 1973;ISBN 978-0-408-70172-3.
Ponomarev VV, and Migarskaya LB. Heats of combustion of some amino-acids. Russ. J. Phys, Chem, (Engl. Transl.) 1960;34:1182–3.
Boerio-Goates J. Heat-capacity measurements and thermodynamic functions of crystalline α-D-glucose at temperatures from 10K to 340K. J. Chem, Thermodynam.1991;23(5):403–9.
Biondi S and Thorpe TA. Clonal propagation of forest tree species. In: Rao, A.N. (ed.): Proceedings COSTED Symposium on Tissue Culture of Economically Important Plants. ANBS, National University, Singapore 1981;pp.197-204.
Moran R. Formulae for determination of chlorophyllous pigments extracted with. N,N-dimethylformamide. Journal of Plant Physiology 1982;69:1376-81.
Sermsiri C, Yaowaphan S, Prakay O and Sontichai C. Shoots induction from in vitro node of teak. King Mongkut's Agricultural Journal 2018;36(2):126-34.
Namita V, Sarita A and Arya ID. Rapid and mass propagation of the economically important desert plant capparis decidua for its afforestation program. Journal of Arid Land Studies 2014;24-1:33-6.
Priyanka S, Swati C, Anita RG, Poonam D, Jyoti R, Kavita S, et al. Effects of adenine sulphate, glutamine and casein hydrolysate on in vitro shoot multiplication and rooting of Kinnow mandarin (Citrus reticulata Blanco). African Journal of Biotechnology Vol 2012;11(92):15852-62.
Umnouysin S, Thepsithar C and Obsuwan K. The comparative study on an appropriate medium for in vitro growth of cassava (Manihot esculenta Cratz). Agricultural Sci. J 2009;40(1):169-72.
Somboon T. Plant physiology. green fence publishing house, Bangkok;1995.206 p.
Murashige T. Plant propagation through tissue culture. Annu. Rev, Plant Physiol 1974;25:135–66.
Waraporn C. Plant growth regulators. Faculty of Science and Technology Nakhon Sawan Rajabhat University;2010.147 p.
Siwapong C. Plant tissue culture. Faculty of Science and Technology Rajabhat Institute Udon Thani, Udon Thani;2003.
Mohanty S, Panda MK, Subudhi E and Nayak S. Plant regeneration from callus culture of curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biol, Plant 2008;52:783-6.
Jala A. Effects of NAA, BA and sucrose on shoot induction and rapid micro-propagation by trimming shoot of Curcuma longa L. Amer. Trans, Eng, Appl, Sci 2011;3(2):101-9.
Vu JCV, Niedz RP and Yelenosky G. Glycerol stimulation of chlorophyll synthesis, embryogenesis andcarboxylation and sucrose metabolism enzymes in nucellar callus of “Hamlin” sweet orange. Plant Cell Tissue Org, Cult 1993;33:75-80.
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2023 มหาวิทยาลัยพะเยา

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ผู้นิพนธ์ต้องรับผิดชอบข้อความในบทนิพนธ์ของตน มหาวิทยาลัยพะเยา ไม่จำเป็นต้องเห็นด้วยกับบทความที่ตีพิมพ์เสมอไป ผู้สนใจสามารถคัดลอก และนำไปใช้ได้ แต่จะต้องขออนุมัติเจ้าของ และได้รับการอนุมัติเป็นลายลักษณ์อักษรก่อน พร้อมกับมีการอ้างอิงและกล่าวคำขอบคุณให้ถูกต้องด้วย







