A Study of Metabolite Profiles and Relationships in the Seaweeds Sargassum polycystum, Caulerpa lentillifera and Gracilaria fisheri
Main Article Content
Abstract
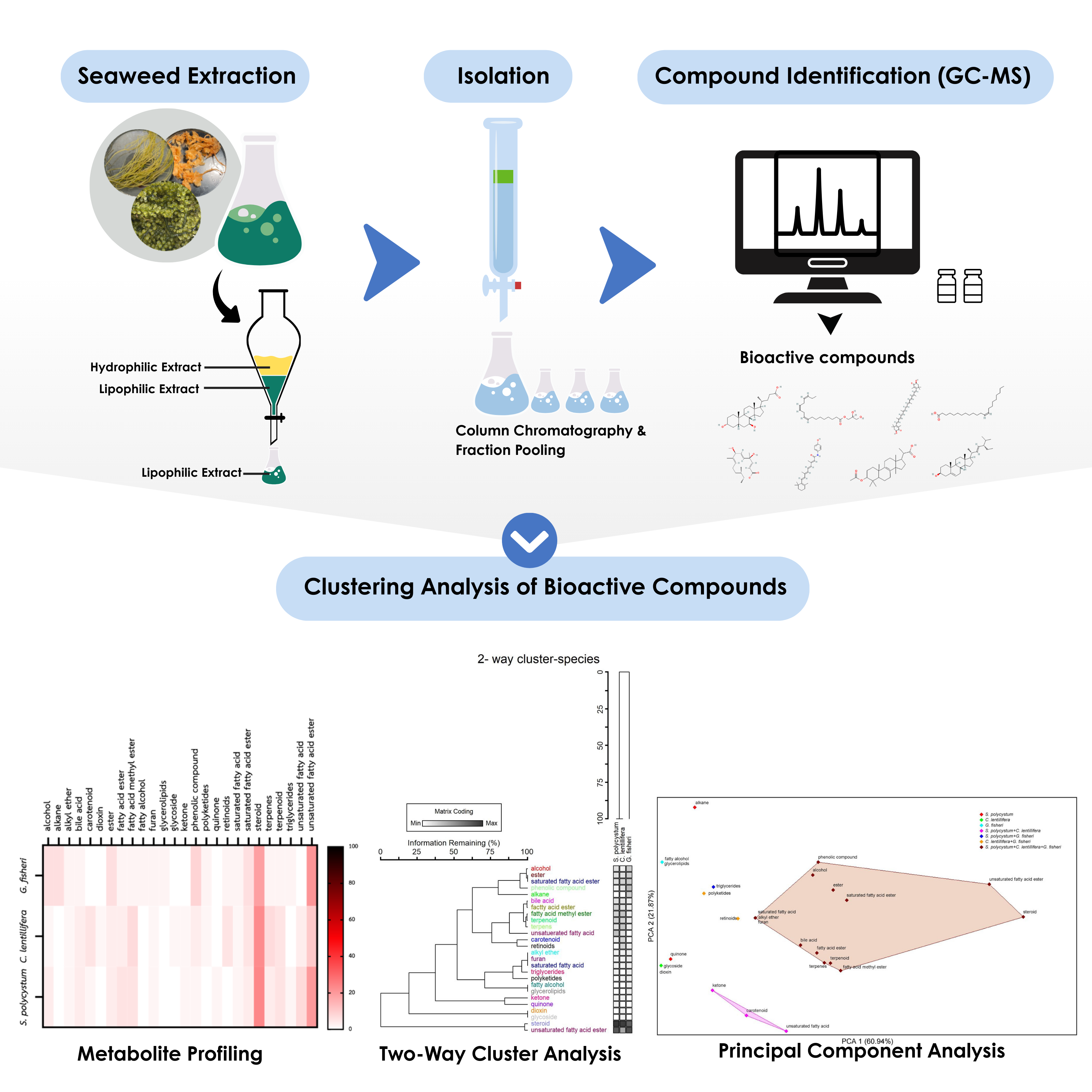
Seaweeds exhibit high biodiversity and produce secondary metabolites with potential applications in various industries. This study aims to analyze the profiles and relationships of metabolites extracted from the lipophilic fractions of three seaweed species commonly found in Thailand, including Sargassum polycystum, Caulerpa lentillifera, and Gracilaria fisheri. The seaweeds were extracted using methanol and fractionated by column chromatography with hexane:ethyl acetate as the solvent system at ratios ranging from 85-95:15-5 (%v/v). Chemical compositions were analyzed using gas chromatography-mass spectrometry (GC-MS), and their relationships were assessed using cluster analysis and Principal Component Analysis (PCA). A total of 112 metabolites were identified and classified into 26 major groups according to their chemical structures. Steroids were the dominant compounds in S. polycystum and C. lentillifera (24% and 23%, respectively), whereas unsaturated fatty acid esters were predominant in G. fisheri (21%). Cluster analysis and PCA revealed similarities in the key metabolite compositions of S. polycystum and C. lentillifera, which were distinct from those of G. fisheri. This solvent system effectively isolated unsaturated fatty acid esters in Fraction 2 of S. polycystum and steroids in Fraction 3 from both C. lentillifera and G. fisheri. This research highlights the potential of these three seaweed species as raw materials for metabolite extraction and demonstrates that metabolite relationship data can be applied to optimize extraction processes, paving the way for product development in various industries.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
เนื้อหาและข้อมูลในบทความที่ลงตีพิมพ์ในวารสารวิชชา มหาวิทยาลัยราชภัฏนครศรีธรรมราช ถือเป็นข้อคิดเห็นและความรับผิดชอบของผู้เขียนบทความโดยตรง ซึ่งกองบรรณาธิการวารสารไม่จำเป็นต้องเห็นด้วยหรือร่วมรับผิดชอบใด ๆ
บทความ ข้อมูล เนื้อหา รูปภาพ ฯลฯ ที่ได้รับการตีพิมพ์ในวารสารวิชชา มหาวิทยาลัยราชภัฏนครศรีธรรมราช ถือเป็นลิขสิทธ์ของวารสารวิชชา มหาวิทยาลัยราชภัฏนครศรีธรรมราช หากบุคคลหรือหน่วยงานใดต้องการนำข้อมูลทั้งหมดหรือส่วนหนึ่งส่วนใดไปเผยแพร่ต่อหรือเพื่อการกระทำการใด ๆ จะต้องได้รับอนุญาตเป็นลายลักษณ์อักษรจากวารสารวิชชา มหาวิทยาลัยราชภัฏนครศรีธรรมราชก่อนเท่านั้น
The content and information in the article published in Wichcha journal Nakhon Si Thammarat Rajabhat University, It is the opinion and responsibility of the author of the article. The editorial journals do not need to agree. Or share any responsibility.
References
ศิริวรรณ ศรีสิทธิ์ และประไพรัตน์ สีพลไกร. (2566). เทอร์พีนอยด์ที่แยกได้จากมะดูก. วารสารวิทยาศาสตร์บูรพา, 28(3), 2087-2100.
สายัณห์ นันชะนะ สุพรรษา ขันธโสภา และนาริสรา วงศ์สิงห์. (2566). ผลของความเข้มแสงต่อการเจริญเติบโตและการผลิตสารสีแคโรทีนอยด์(carotenoids) ของสาหร่ายสายพันธุ์ Chlorococcum sp. TISTR 8266. วารสารวิชชา มหาวิทยาลัยราชภัฏนครศรีธรรมราช, 42(1), 120-134, doi: https://doi.org/10.65217/wichchajnstru.2023.v42i1.256500.
Batool, A. and Menaa, F. (2020). Concentration and purification of seaweed components by chromatography methods. In Tiwari, B.K. and Troy, D. (Eds.). Sustainable seaweed technologies, pp. 315-370. Amsterdam: Elsevier.
Bialek, A., Bialek, M., Jelinska, M. and Tokarz, A. (2016). Fatty acid profile of new promising unconventional plant oils for cosmetic use. International Journal of Cosmetic Science, 38(4), 382-388, doi: https://doi.org/10.1111/ics.12301.
Buathong, R., Schindler, F., Schinnerl, J., Valant-Vetschera, K., Bacher, M., Potthast, A., Rosenau, T. and Vajrodaya, S. (2019). Uncommon fatty acids, iridoids and other secondary metabolites from the medicinal plant species Ixora cibdela Craib (Rubiaceae). Phytochemistry Letters, 33, 77-80, doi: https://doi.org/10.1016/j.phytol.2019.07.011.
Chan, C.X. and Bhattacharya, D. (2010). The origin of plastids. Nature Education, 3(9), 84.
Chhetri, B.K., Mojib, N., Moore, S.G., Delgadillo, D.A., Burch, J.E., Barrett, N.H., Gaul, D.A., Marquez, L., Soapi, K., Nelson, H.M., Quave, C.L. and Kubanek, J. (2023). Cryptic chemical variation in a marine red alga as revealed by nontargeted metabolomics. ACS Omega, 8(15), 13899-13910, doi: https://doi.org/10.1021/acsomega.3c00301.
Coppejans, E., Prathep, A., Leliaert, F., Lewmanomont, K. and De Clerck, O. (2010). Seaweeds of Mu Ko Tha Lae Thai (SE Thailand): Methodologies and field guide to the dominant species (Vol. 11). Bangkok: Biodiversity Research and Training Program.
Cornish, M.L. and Garbary, D.J. (2010). Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae, 25(4), 155-171, doi: https://doi.org/10.4490/algae.2010.25.4.155.
Elbahnaswy, S. and Elshopakey, G.E. (2024). Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: A review. Fish Physiology and Biochemistry, 50(1), 97-126, doi: https://doi.org/10.1007/s10695-022-01167-0.
Fan, M., Yuan, S., Li, L., Zheng, J., Zhao, D., Wang, C., Wang, H., Liu, X. and Liu, J. (2023). Application of terpenoid compounds in food and pharmaceutical products. Fermentation, 9(2), 119, doi: https://doi.org/10.3390/fermentation9020119.
Farage, M.A., Miller, K.W., Elsner, P. and Maibach, H.I. (2007). Structural characteristics of the aging skin: A review. Cutaneous and Ocular Toxicology, 26(4), 343-357, doi: https://doi.org/10.1080/15569520701622951.
Ferreira, V.F., de Carvalho, A.S., Ferreira, P.G., Lima, C.G.S. and da Silva, F.C. (2021). Quinone-based drugs: An important class of molecules in medicinal chemistry. Medicinal Chemistry, 17(10), 1073-1085, doi: https://doi.org/10.2174/1573406416666201106104756.
Imokawa, G. (2019). The xanthophyll carotenoid astaxanthin has distinct biological effects to prevent the photoaging of the skin even by its postirradiation treatment. Photochemistry and Photobiology, 95(2), 490-500, doi: https://doi.org/10.1111/php.13039.
Ito, K., Sakata, K., Date, Y. and Kikuchi, J. (2014). Integrated analysis of seaweed components during seasonal fluctuation by data mining across heterogeneous chemical measurements with network visualization. Analytical Chemistry, 86(2), 1098-1105, doi: https://doi.org/10.1021/ac402869b.
Kumarasinghe, H.S. and Gunathilaka, M.D.T.L. (2024). A systematic review of fucoxanthin as a promising bioactive compound in drug development. Phytochemistry Letters, 61, 52-65, doi: https://doi.org/10.1016/j.phytol.2024.03.009.
McCune, B. and Mefford, M.J. (2016). PC-ORD: Multivariate analysis of ecological data. Version 7. Retrieved 27 November 2024, from: https://static1squarespace.com/static/58f588c93e00be17785ced5d/t/5bcccc404785d3d9aac3e266/1540148334020/PBooklet7.pdf.
Millman, R.B. and Ross, E.J. (2003). Steroid and nutritional supplement use in professional athletes. The American Journal on Addictions, 12(s2), S48-S54, doi: https://doi.org/10.1111/j.1521-0391.2003.tb00556.x.
Naikwadi, P.H., Phatangare, N.D. and Mane, D.V. (2023). Active anti-inflammatory potency of γ-sitosterol from Woodfordia floribunda Salisb. The Journal of Plant Science Research, 38(2), 691-700, doi: https://doi.org/10.32381/JPSR.2022.38.02.23.
Napiroon, T., Tanruean, K., Poolprasert, P., Bacher, M., Balslev, H., Poopath, M. and Santimaleeworagun, W. (2021). Cannabinoids from inflorescences fractions of Trema orientalis (L.) Blume (Cannabaceae) against human pathogenic bacteria. PeerJ, 9, e11446, doi: https://doi.org/10.7717/peerj.11446.
Nazarudin, M.F., Alias, N.H., Balakrishnan, S., Wan Hasnan, W.N.I., Noor Mazli, N.A.I., Ahmad, M.I., Md Yasin, I.-S., Isha, A. and Aliyu-Paiko, M. (2021). Chemical, nutrient and physicochemical properties of brown seaweed, Sargassum polycystum C. Agardh (Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. Molecules, 26(17), 5216, doi: https://doi.org/10.3390/molecules26175216.
Nazarudin, M.F., Isha, A., Mastuki, S.N., Ain, N.M., Mohd Ikhsan, N.F., Abidin, A.Z. and Aliyu-Paiko, M. (2020). Chemical composition and evaluation of the α-glucosidase inhibitory and cytotoxic properties of marine algae ulva intestinalis, Halimeda macroloba, and Sargassum ilicifolium. Evidence-Based Complementary and Alternative Medicine, 2020(1), 2753945, doi: https://doi.org/10.1155/2020/2753945.
Ng, P.K., Lin, S.M., Lim, P.E., Hurtado, A.Q., Phang, S.M., Yow, Y.Y. and Sun, Z. (2017). Genetic and morphological analyses of Gracilaria firma and G. changii (Gracilariaceae, Rhodophyta), the commercially important agarophytes in western Pacific. Plos One, 12(7), e0182176, doi: https://doi.org/10.1371/journal.pone.0182176.
Noiraksar, T. and Ajisaka, T. (2009). Taxonomy and distribution of Sargassum (Phaeophyceae) in the Gulf of Thailand. In Proceedings of the 19th International Seaweed Symposium (pp. 513-527). Dordrecht: Springer.
Price, I.R. (2011). A taxonomic revision of the marine green algal genera Caulerpa and Caulerpella (Chlorophyta, Caulerpaceae) in northern (tropical and subtropical) Australia. Australian Systematic Botany, 24(3), 137-213, doi: https://doi.org/10.1071/SB10033.
Pulikkottil, B.J., Dauwe, P., Daniali, L. and Rohrich, R.J. (2013). Corticosteroid use in cosmetic plastic surgery. Plastic and Reconstructive Surgery, 132(3), 352e-360e, doi: https://doi.org/10.1097/PRS.0b013e31829acc60.
Sayuti, N.H., Muhammad Nawawi, K.N., Goon, J.A., Mokhtar, N.M., Makpol, S. and Tan, J.K. (2023). A review of the effects of fucoxanthin on NAFLD. Nutrients, 15(8), 1954, doi: https://doi.org/10.3390/nu15081954.
Sundarraj, S., Thangam, R., Sreevani, V., Kaveri, K., Gunasekaran, P., Achiraman, S. and Kannan, S. (2012). γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. Journal of Ethnopharmacology, 141(3), 803-809, doi: https://doi.org/10.1016/j.jep.2012.03.014.
Tahir, N., Qader, K., Azeez, H. and Rashid, J. (2018). Inhibitory allelopathic effects of Moringa oleifera Lamk plant extracts on wheat and Sinapis arvensis L. Allelopathy Journal, 44, 35-48, doi: https://doi.org/10.26651/allelo.j./2018-44-1-1152.
Tapotubun, A.M., Rieuwpassa, F., Supratman, U. and Setha, B. (2019). Effect of different drying methods on phytochemical content of Caulerpa lentillifera from Kei islands. International Journal of ChemTech Research, 12(6), 109-115, doi: https://doi.org/10.20902/IJCTR.2019.120614.
Teramukai, K., Kakui, S., Beppu, F., Hosokawa, M. and Miyashita, K. (2020). Effective extraction of carotenoids from brown seaweeds and vegetable leaves with edible oils. Innovative Food Science and Emerging Technologies, 60, 102302, doi: https://doi.org/10.1016/j.ifset.2020.102302.
Torres, P., Santos, J.P., Chow, F. and dos Santos, D.Y.A.C. (2019). A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Research, 37, 288-306, doi: https://doi.org/10.1016/j.algal.2018.12.009.
Ullah, T., Muhammad, Z., Shah, I. A., Bourhia, M., Nafidi, H.-A., Mohammad Salamatullah, A. and Ali Younous, Y. (2024). Multivariate analysis of the summer herbaceous vegetation and environmental factors of the sub-tropical region. Scientific Reports, 14(1), 15657, doi: https://doi.org/10.1038/s41598-024-63780-8.
Wu, Y., Gao, H., Wang, Y., Peng, Z., Guo, Z., Ma, Y., Zhang, R., Zhang, M., Wu, Q. and Xiao, J. (2022). Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. Journal of Food Science, 87(3), 968-981, doi: https://doi.org/10.1111/1750-3841.16051.
Yin, S., Niu, L., Shibata, M., Liu, Y. and Hagiwara, T. (2022). Optimization of fucoxanthin extraction obtained from natural by-products from Undaria pinnatifida stem using supercritical CO2 extraction method. Frontiers in Nutrition, 9, 981176, doi: https://doi.org/10.3389/fnut.2022.981176.
Zhang, K., Li, T., Shan, X., Lu, R., Zhang, S. and Xu, H. (2021). Cholesterol: Bioactivities, structural modification, mechanisms of action, and structure-activity relationships. Mini Reviews in Medicinal Chemistry, 21(14), 1830-1848, doi: https://doi.org/10.2174/1389557521666210105123320.
Zhang, L.-L. and Lin, Y.-M. (2008). Tannins from Canarium album with potent antioxidant activity. Journal of Zhejiang University SCIENCE B, 9(5), 407-415, doi: https://doi.org/10.1631/jzus.B0820002.